NCERT Exemplar Class 11 Chemistry Chapter 12 Organic Chemistry: Some Basic Principles and Techniques are part of NCERT Exemplar Class 11 Chemistry. Here we have given NCERT Exemplar Class 11 Chemistry Chapter 12 Organic Chemistry: Some Basic Principles and Techniques.
NCERT Exemplar Class 11 Chemistry Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
Multiple Choice Questions
Single Correct Answer Type
Q1. Which of the following^ the correct IUPAC name?
(a) 3-Ethyl-4,4-dimethylheptane
(b) 4,4-Dimethyl-3-ethylheptane
(c) 5-Ethyl-4,4-dimethylheptane
(d) 4,4-Bis(methyl)-3-ethylheptane
Sol: (a) While writing IUPAC name, the alkyl groups are written in alphabetical order. Thus lower locant 3 is assigned to ethyl. Prefix, di, tri, and tetra are not included in alphabetical order.
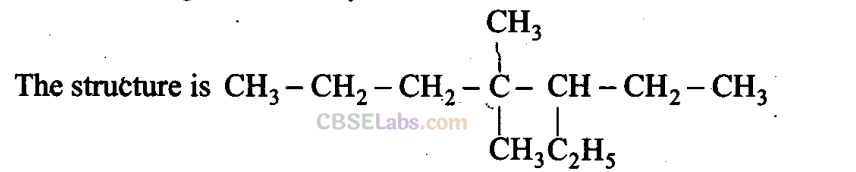
Q2. The IUPAC name for
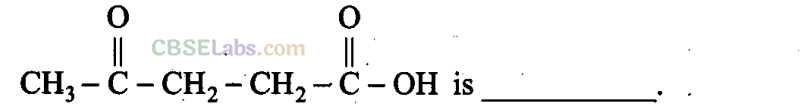
(a) 1-Hydroxypentane-l, 4-dione
(b) 1,4-Dioxopentanol
(c) l-Carboxybutan-3-one
(d) 4-Oxopentanoic acid
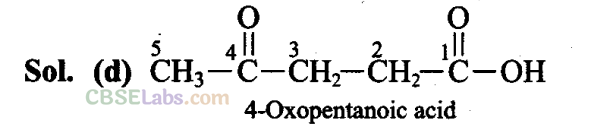
When more than one functional group lie in the main chain, nomenclature is done according to that functional group which has higher priority. Carboxylic acid (-COOH) has more priority than the keto group (>C = O).
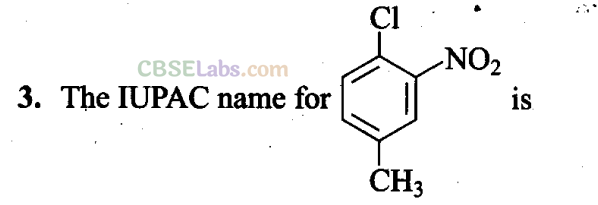
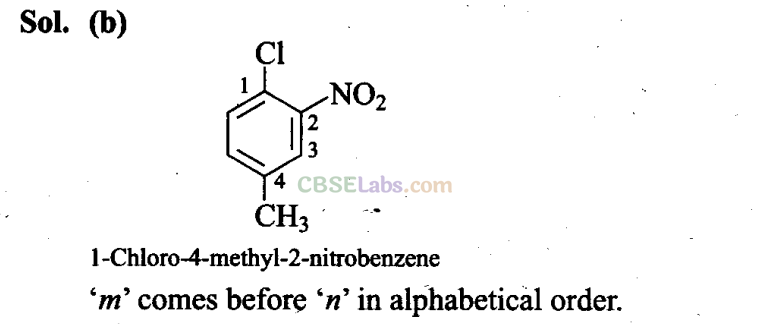
(a) 1 -Chloro-2-nitro-4-methylbenzene
(b) l-Chloro-4-methyl-2-nitrobenzene
(c) 2-Chloro-1 -nitro-5-methylbenzene
(d) m-Nitro-p-chlorotoluene
Q4. Electronegativity of carbon atoms depends upon their state of hybridization. In which of the following compounds, the carbon marked with asterisk is most electronegative?
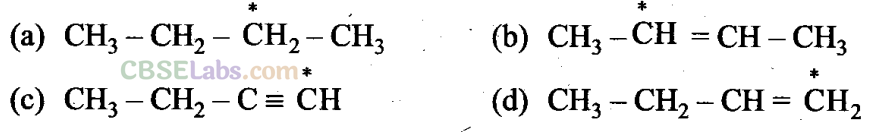
Sol: (c) Electronegativity increases as the state of hybridization changes from sp3 to sp2 and sp2 to sp. Thus, sp hybridized carbon has the highest electronegativity.
Q5. In which of the following, functional group isomerism is not possible?
(a) Alcohols
(b) Aldehydes
(c) Alkyl halides
(d) Cyanides
Sol: (c) Alkyl halides do not show functional isomerism. Alcohols and ethers, aldehydes and ketones, cyanides and isocyanides are functional isomers.
Q6. The fragrance of flowers is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour in vapour phase. A suitable method for the extraction of these oils from the flowers is
(a) distillation
(b) crystallisation
(c) distillation under reduced pressure
(d) steam distillation.
Sol: (d) Essential oils are insoluble in water, soluble in steam and have high vapour pressure. Therefore, they can be separated by steam distillation.
Q7. During hearing of a court case, the judge suspected that some changes in the documents had been carried out. He asked the forensic department to check the ink used at two different places. According to you, which technique can give the best results?
(a) Column chromatography (b) Solvent extraction
(c) Distillation (d) Thin layer chromatography
Sol:(d) Thin layer chromatography (TLC) is used to separate the pigments present in ink.
Q8. The principle involved in paper chromatography is
(a) adsorption (b) partition
(c) solubility (d) volatility
Sol:(b) In paper chromatography, separation of the components of a mixture depends upon their partitioning between water held in the stationary phase (i.e. adsorbent paper) and the liquid present in the mobile phase.
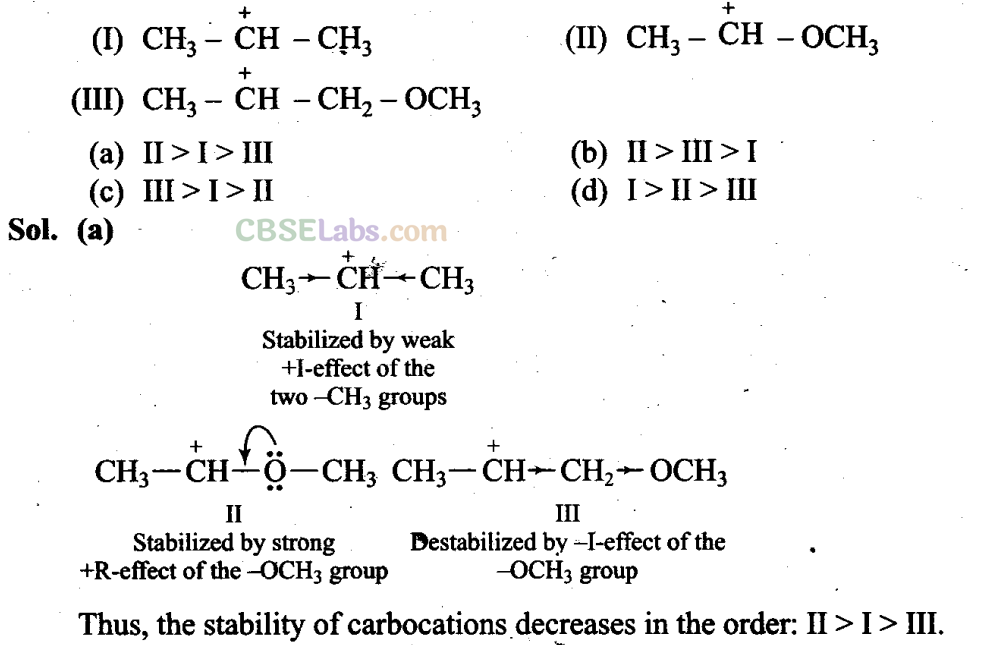
Q9. What is the correct order of decreasing stability of the following cations?
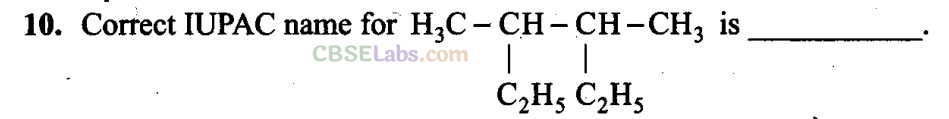
(a) 2-Ethyl-3-methylpentane
(b) 3,4-Dimethylhexane
(c) 2-sec-Butylbutane
(d) 2,3-Dimethylbutane
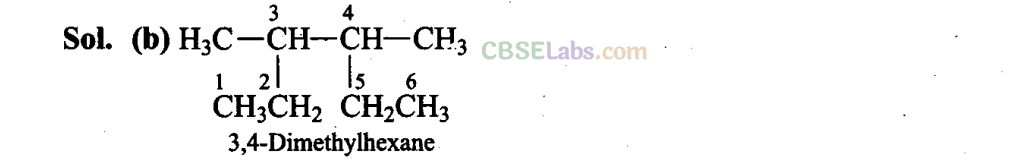
Q11. In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?

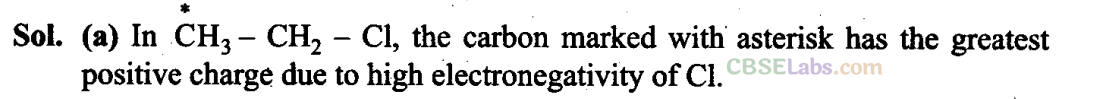
Q12. Ionic species are stabilized by the dispersal of charge. Which of the following carboxylate ions is the most stable?

Sol: (d) In all the given carboxylate ions, the negative charge is dispersed which stabilizes these ions. Here, the negative charge is dispersed by two factors, i.e., +R-effect of the carboxylate ion (conjugation) and -I-effect of the halogens.
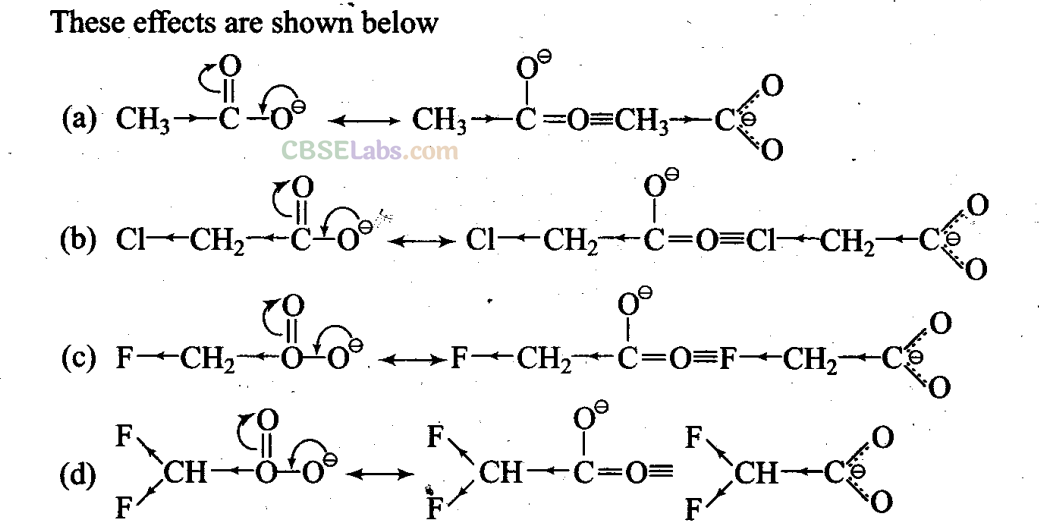
It is evident from the above structures that +R-effect is common in all the four ions. Therefore, overall dispersal of negative charge depends upon the number of halogen atoms and electronegativity. Since F has the highest electronegativity and two F-atoms are present in option (d), thus, dispersal of negative charge is maximum in option (d).
Q13. Electrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
H3C-HC = CH2 + H+→ ?
(a) 2°Carbanion
(b) 1° Carbocation
(c) 2° Carbocation
(d) l°Carbanion
Sol: (c) When the electrophile attacks CH3 – CH = CH2, delocalisation of electrons can take place in two possible ways
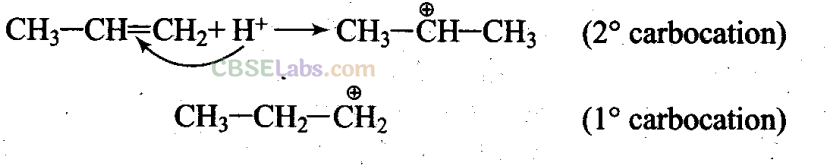
As 2° carbocation is more stable than 1° carbocation, the first addition is more feasible.
Q14. Covalent bonds can undergo fission in two different ways. The correct representation involving the heterolytic fission of CH3 – Br is
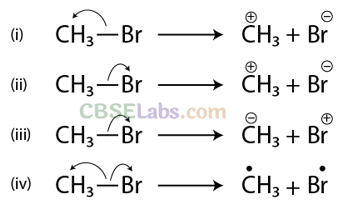
Sol: (b) Arrow denotes the direction of movement of electrons
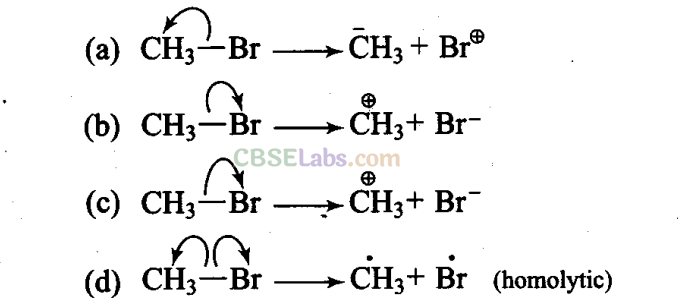
Since, Br is more electronegative than carbon, hence heterolytic fission occurs in such a way that CH3 gets the positive charge and Br gets the negative charge. Thus, option (b) is correct.
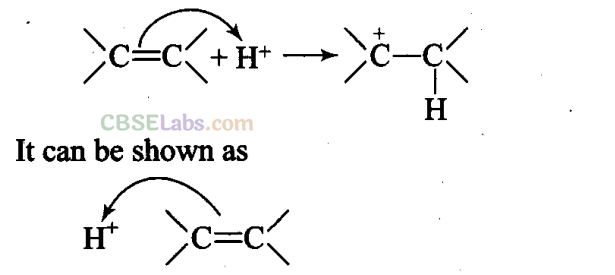
Q15. The addition of HC1 to an alkene proceeds in two steps. The first step is the attack of H+ ion to >C = C< portion which can be shown as
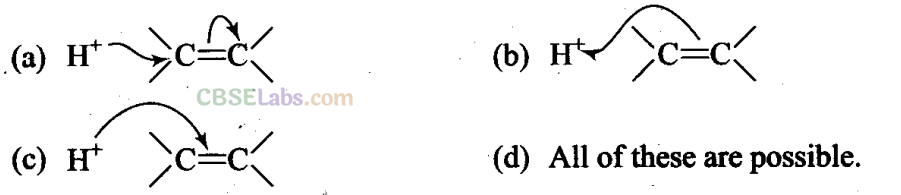
Sol: (b) Since double bond is a source of electrons and the charge flows from source of more electron density, therefore, n electrons of the double bond attack the proton.
More than One Correct Answer Type
Q16. Which of the following compounds contain all the carbon atoms in the same hybridization state?

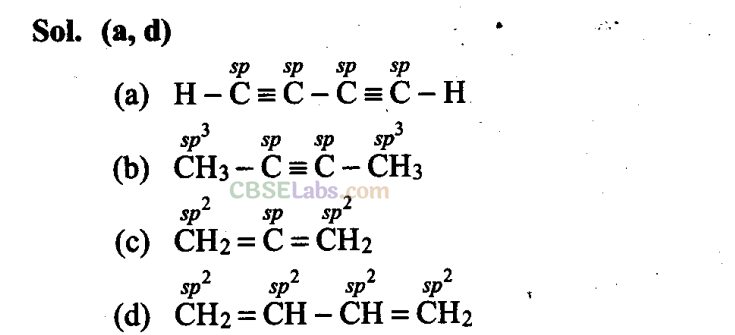
Q17. In which of the following representations given below spatial arrangement of group/atom is different from that given in structure ‘A’?
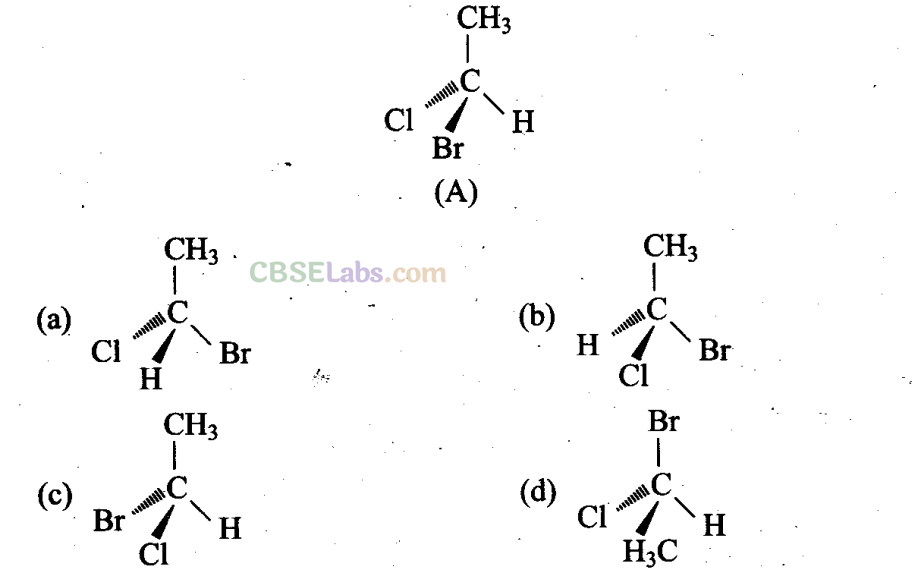
Sol: (a, c, d) Different groups are present towards and away from the observer.
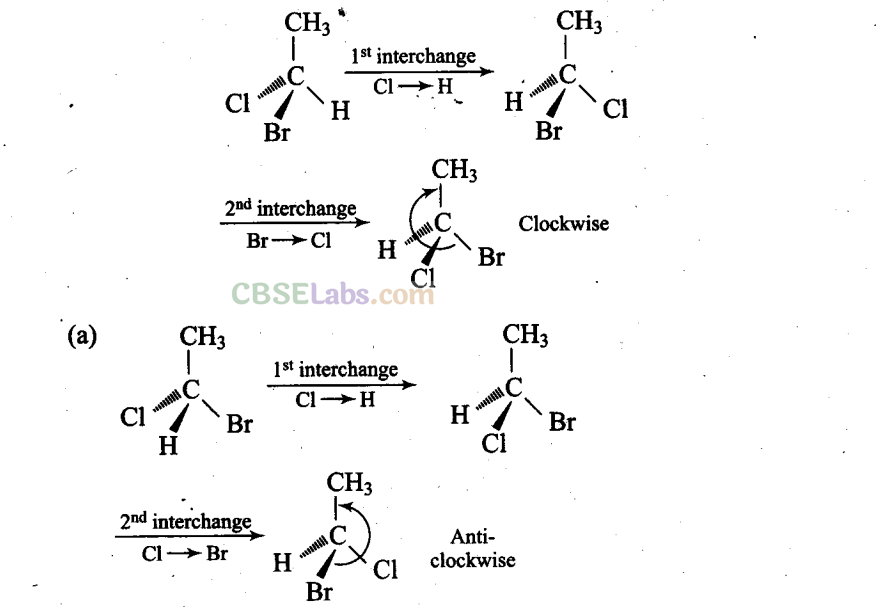
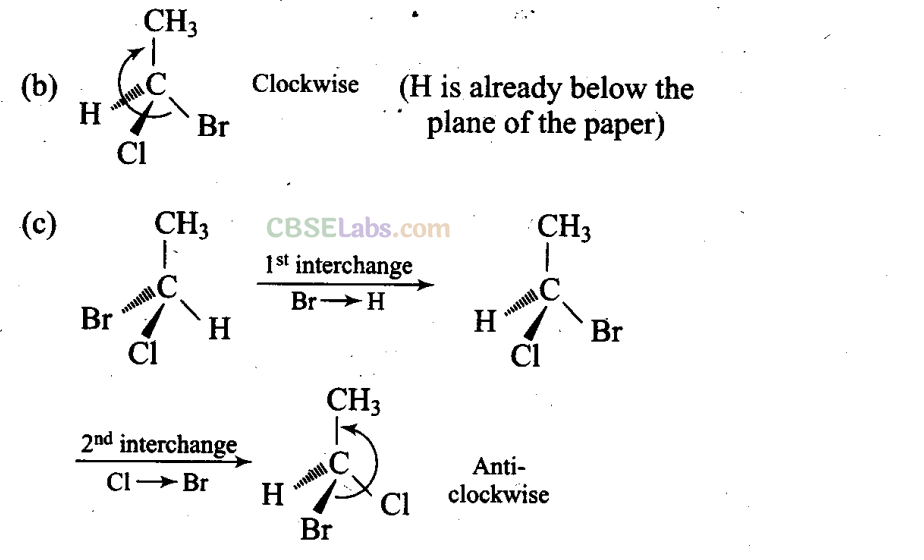
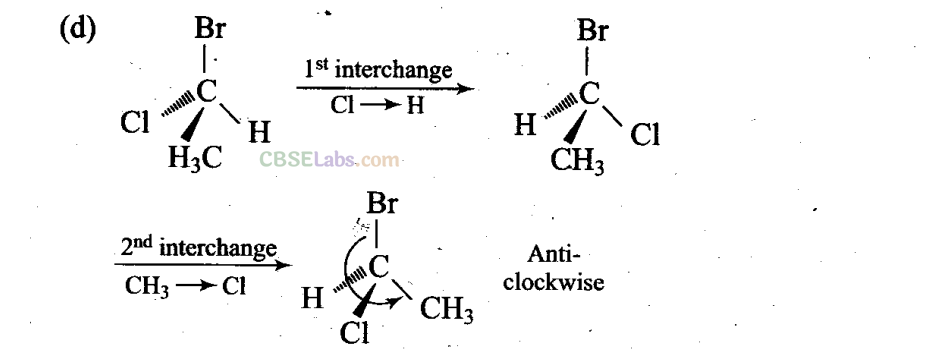
(a), (c) and (d) have different (anticlockwise) spatial arrangements of atoms or groups of atoms than that given in structure A (clockwise).
Q18. Electrophiles are electron seeking species. Which of the following groups contains only electrophiles?
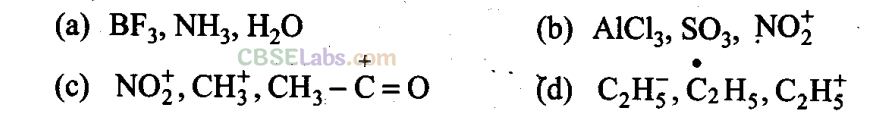
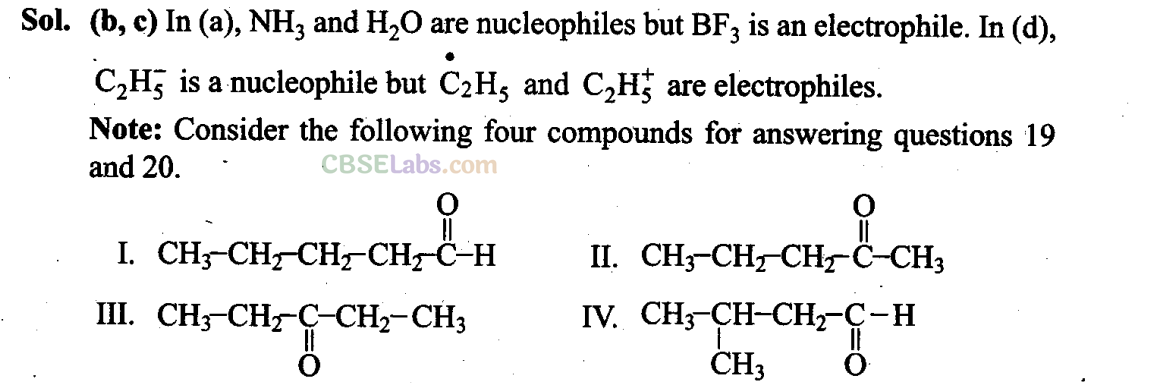
Q19. Which of the above pairs are position isomers?
(a) I and II
(b) II and III
(c) II and IV
(d) III and IV
Sol: (b) II and III are position isomers as they differ in the position of -C- group.
Q20. Two or more compounds having same the molecular formula but different functional groups are called functional isomers. Which of die following pairs are not functional group isomers?
(a) II and III (b) II and IV
(c) I and IV (d) I and II
Sol: (a, c) (a) II and III have the same functional group.
(c) I and IV have the same functional group.
Q21. Nucleophile is a species that should have
(a) a pair of electrons to donate
(b) positive charge
(c) negative charge
(d) electron deficient species
Sol: (a, c) Nucleophile (nucleus-loving) is a chemical species that donates an, electron pair to an electrophile (electron-loving). Hence, a nucleophile should
have either a negative charge or an electron pair to donate. Thus, options (a) r and (c) are correct.
Q22. Hyperconjugation involves delocalization of .
(a) electrons of carbon-hydrogen σ bond of an alkyl group directly attached to an atom of unsatUrated system.
(b) electrons of carbon-hydrogen σ bond of alkyl group directly attached to the positively charged carbon atom.
(c) π-electrons of carbon-carbon bond.
(d) lone pair of electrons.
Sol: (a, b) Hyperconjugation, also known as sigma-pi conjugation, is the delocalization of sigma electrons. Presence of -H with respect to double bond, triple bond or carbon containing positive charge (in carbonium ion) or unpaired electron (in free radical) is a condition required for hyperconjugation.
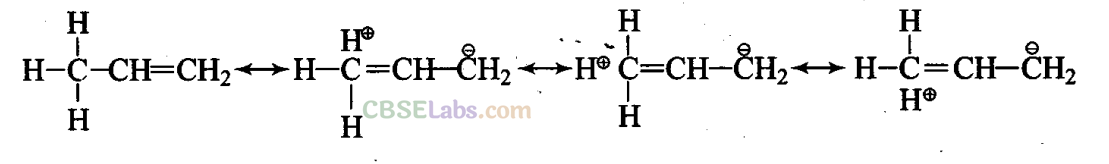
Short Answer Type Questions
Note: Consider structures I to VII and answer Questions 23-26.
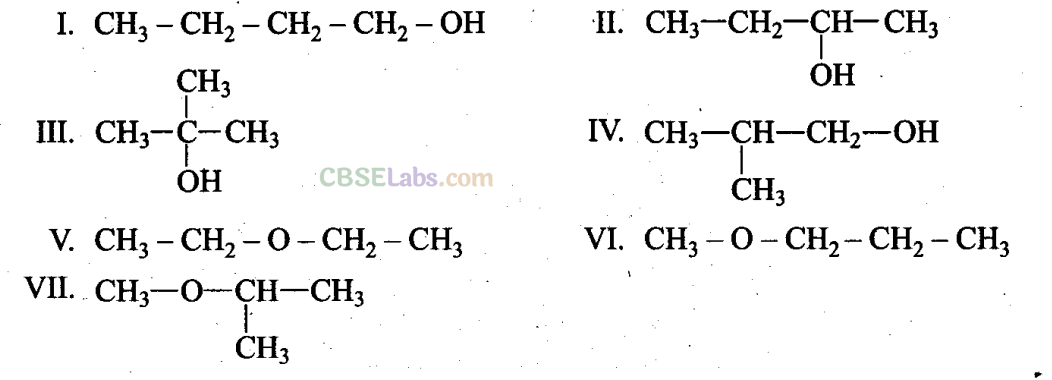
Q23. Which of the above compounds form pairs of metamers?
Sol: V and VI, VI and VII and V and VII form pairs of metamers because they have different alkyl chains on either side of ethereal oxygen (-O-).
Q24. Identify the pairs of compounds which are functional group isomers.
Sol: Compounds I to IV, i.e., alcohols, and V to VII, i.e., ethers, are functional group isomers with molecular formula C4H10O and different functional groups (-OH in I to IV and -O- in V to VII).
Hence, I and V, I and VI, I and VII; II and V, II and VI, II and VII; HI and V, IH and VI; IH and VII; IV and V, IV and VI, IV and VH are functional group isomers.
Q25. Identify the pairs of compounds that represent position isomerism.
Sol: I and II, III and IV, VI and VII are position isomers due to different positions of -OH group and -O- group.
Q26. Identify the pairs of compounds that represent chain isomerism.
Sol: I and III, I and IV, II and III, II and IV.
Q27. For testing halogens in an organic compound with AgN03 solution, sodium extract (Lassaigne’s extract) is acidified with dilute HN03. What will happen if a student acidifies the extract with dilute H2S04 in place of dilute HN03?
Sol: On adding dilute H2S04 for testing halogens in an organic compound with AgN03, white precipitate of Ag2S04 is formed. This will interfere with the test of chlorine and this Ag2S04 may be mistaken for white precipitate of chlorine as AgCl. Hence, dilute HN03 should be used instead of dilute H2S04.
Q28. What is the hybridization of each carbon in H7C = C = CH7?
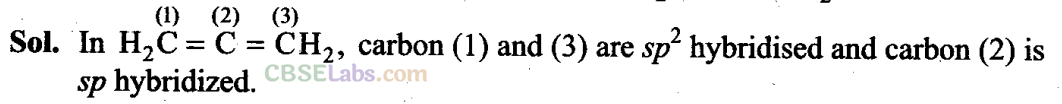
Q29. Explain how is the electronegativity of carbon atoms related to their state of hybridization in an organic compound?
Sol: Electronegativity increases with increasing s-character. This is because s-electrons are more strongly attracted by the nucleus than p-electrons.
sp3 – 25% s-character, 75% P-character
sp2 – 33% s-character, 67% P-character –
sp – 50% s-character, 50% P-character
Hence, the order of electronegativity is sp3 < sp2 < sp
Q30. Show the polarization of carbon-magnesium bond in the following structure.
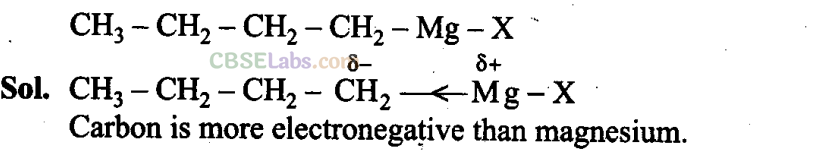
Q31. Compounds with same molecular formula but differing in their structures are said to be structural isomers. What type of structural isomerism is shown by
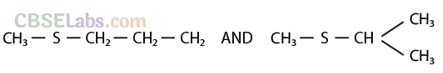
Sol: They show position isomerism.
Q32. Which of the following selected chains is correct to name the given compound according to IUPAC system?
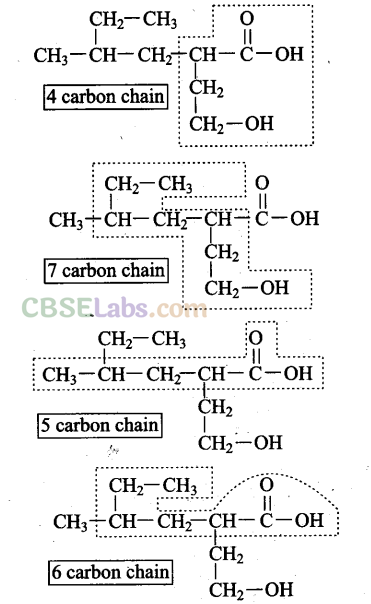
Sol: The 4 carbon chain is correct*according to the IUPAC system since it contains both the functional groups. The other three carbon chains are incorrect since none of them contains both the functional groups.
Q33. In DNA and RNA, nitrogen atom is present in the ring system. Can Kjeldahl method be used for the estimation of nitrogen present in these? Give reasons.
Sol: No. DNA and RNA have nitrogen in the heterocyclic rings which cannot be removed as ammonia.
Kjeldahl method cannot be used to estimate nitrogen present in rings, azo and nitro groups as nitrogen present in these cannot be converted into ammonium sulphate.
Q34.If a liquid compound decomposes at its boiling point, which method(s) will you choose for its purification? It is known that the compound is stable at low pressure, steam volatile and insoluble in water.
Sol: Steam distillation can be used for its purification. This method is applied to separate substances which are steam volatile and immiscible with water. Note: Answer Questions 35-38 on the basis of information given below: “Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom, involvement of neighbouring groups in hyperconjugation and resonance.”

Q36. Which of the following ions is more stable? Use resonance to explain your Answer
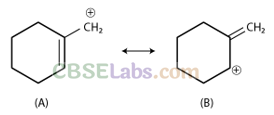
Sol: Structure (A) is more stable due to resonance. Structure (B) is non-planar and hence it does not undergo resonance. Double bond is more stable within the ring in comparison to outside the ring.
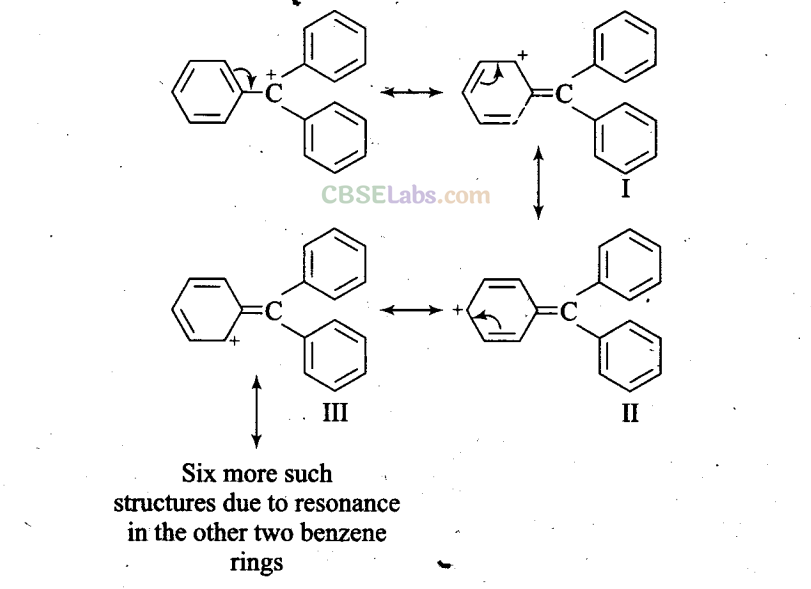
Q37. The structure of triphenylmethyl cation is given here. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation
Sol: Triphenylmethyl cation is a tertiary carbocation which can show nine possible canonical structures and hence is very stable.
Q38. Write the structures of various carbocations that can be obtained from 2-methylbutane. Arrange these carbocations. in order of increasing stability.
Sol: 2-Methylbutane has four possible carbocations
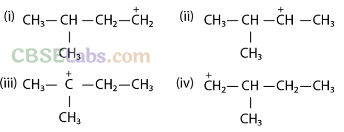
Stability of carbocation increases in the order 1° < 2° < 3°. Out of I and IV, IV is more stable than I because in IV, CH3 group is at a-carbon and in I, it is at β-carbon and +I-effect decreases with distance
Q39. Three students, Manish, Ramesh and Rajni, were determining the extra elements present in an organic compound given by their teacher. They prepared the Lassaigne’s extract (LE) independently by the fusion of the compound with sodium metal. Then they added solid FeS04 and dilute sulphuric acid to a part of Lassaigne’s extract. Manish and Rajni obtained Prussian blue colour bit Ramesh got red colour. Ramesh repeated the test with the same Lassaigne’s extract, but again got red colour only. They were surprised and went to their teacher and told him about their observation. Teacher asked them to think over the reason for this. Can you help them by giving the reason for this observation? Also, write the chemical equations to explain the formation of compounds of different colours.
Sol: In the Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of
ferri ferrocyanide.
6NaCN + FeS04→ Na4[Fe(CN)6] + Na2S04
3Na4[Fe(CN)6] + 2Fe2(S04)3 → Fe4[Fe(CN)6]3 + 6Na2S04
In compounds containing nitrogen and sulphur together, the sodium metal should be in slight excess otherwise in Lassaigne’s test, sodium thiocyanate (NaCNS) is formed which gives red colour with Fe3+ ions and decomposes as follows:
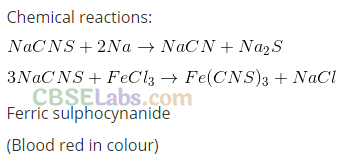
On the basis of above results, it is clear that Ramesh used less sodium and hence NaSCN was formed in the Lassaigne’s extract which gave red colouration due to Fe(SCN)3 formation while Manish and Rajni used excess sodium and hence, NaCN was formed in the Lassaigne’s extract which gave Prussian blue colour of Fe4[Fe4(CN)6].
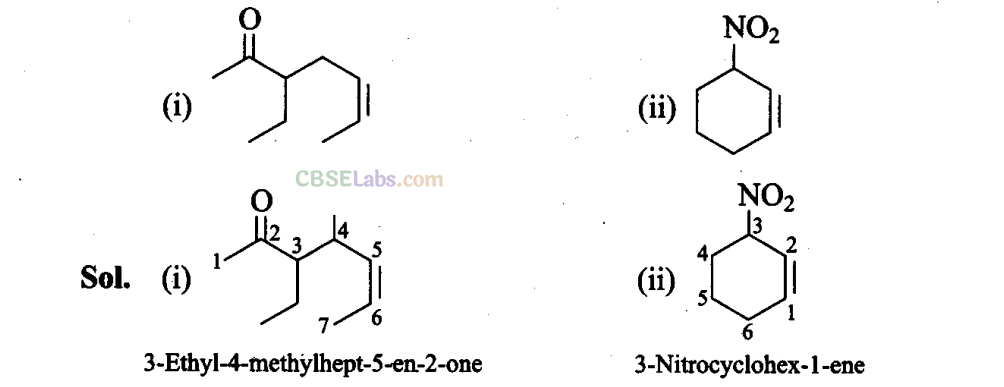
Q40. Name the compounds whose line formulae are given below:
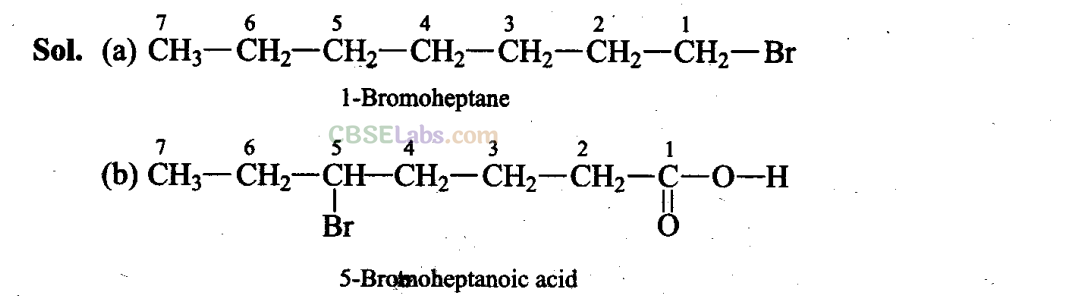
Q41. Write structural formulae for compounds named as
(a) 1-Bromoheptane
(b) 5-Bromoheptanoic acid
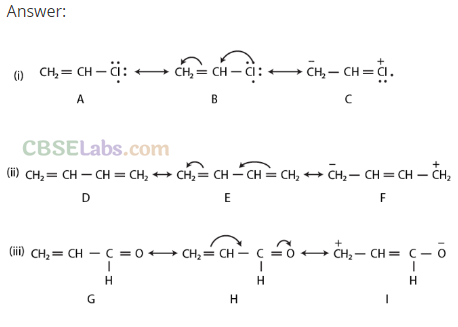
Q42. Draw the resonance structures of the following compounds:

Q43. Identify the most stable species in the following set of ions giving reasons:
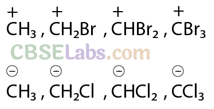
Q44. Give three points of differences between inductive effect and resonance effect.
Sol:
| Inductive effect | Resonance effect |
| (1) This effect involves
displacement of σ-electrons. |
The resonance involves displacement of π-electrons or lone pair of electrons. |
| (2) It operates in saturated compounds. | It operates only in unsaturated conjugated systems. |
| (3) This effect moves up to three • carbon atoms and becomes
negligible from fourth carbon atom onwards. |
This effect moves all along the length of the conjugated system. |
| (4) This effect causes slight drift of σ-electrons towards the more electronegative atom and hence only partial charges (δ + and δ -) are developed. | This results in complete transfer of electrons and hence full +ve and -ve charges are developed. |
Q45. Which of the following compounds will not exist as resonance hybrid? Give reason for your answer.
(a) CH3OH
(b) R-CONH2
(c) CH3CH = CHCH2NH2
Sol: (a) CH3OH does not contain -electrons, hence, it cannot exist as resonance hybrid.
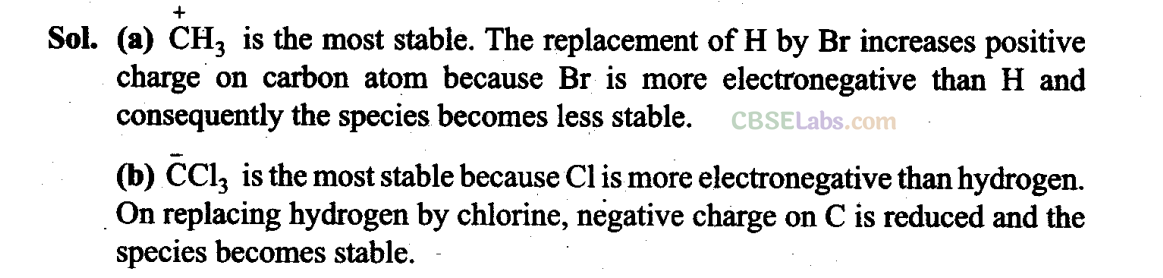
(b) Due to the presence of -electrons in C = O bond and lone pair of electrons on N, amide can be represented by the following resonating structures.
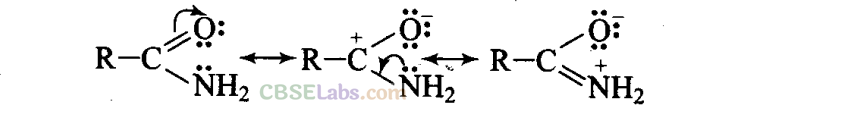
(c) CH3CH = CHCH2NH2: Since lone pair of electrons on nitrogen atom is not conjugated with the -electrons, therefore, resonance is not possible.
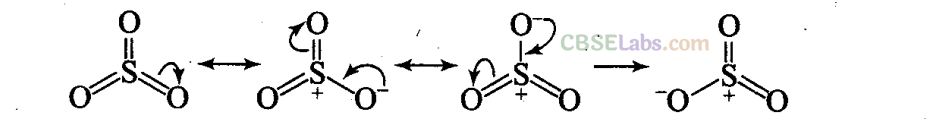
Q46. Why does S03 act as an electrophile?
Sol: Three highly electronegative oxygen atoms are attached to sulphur atom. This makes sulphur atom electron deficient. Due to resonance, sulphur also acquires positive charge. Both these factors make S03 an electrophile.
Q47. Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
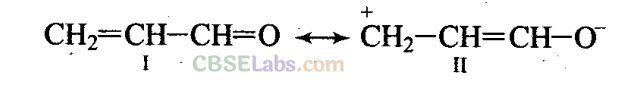

Q48. By mistake, an alcohol (boiling point 97°C) was mixed with a hydrocarbon (boiling point 68°C). Suggest a suitable method to separate the two compounds. Explain the reason for your choice.
Sol: Simple distillation can be used because the two compounds have a difference of more than 20° in their boiling points and therefore, both the liquids can be distilled without any decomposition.
Q49. Which of the two structures (A) and (B) given below is more stabilized by resonance? Explain.
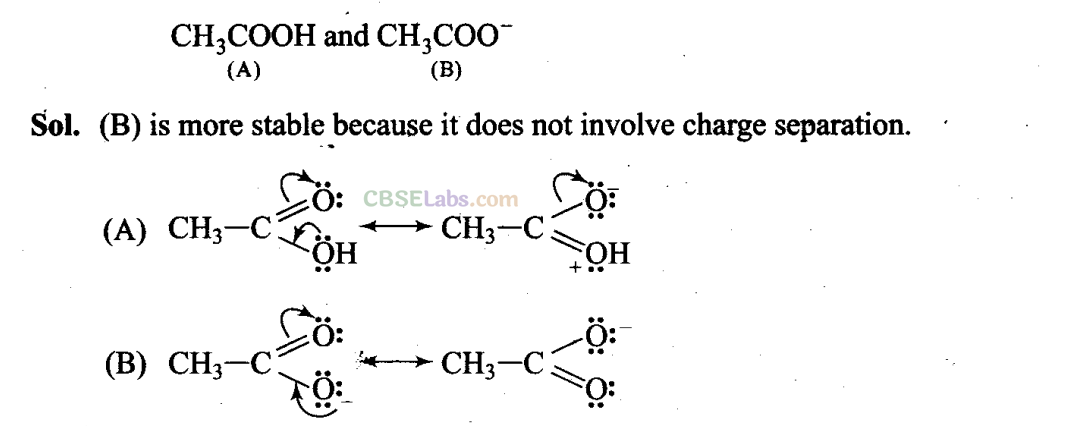
Matching Column Type Questions
In the following questions more than one correlation is possible between options of column I and II. Make as many correlations as you can.
Q50. Match the type of mixture of compounds in Column I with the technique of separation/purification given in column II.
| Column I | Column II |
| (a) Two solids which have different solubilities in a solvent and which do not undergo reaction when dissolved in it. | (1) Steam distillation |
| (b) Liquid that decomposes at its boiling point | (2) Fractional distillation |
| (c) Steam volatile liquid | (3) Simple distillation |
| (d) Two liquids which have boiling points close to each other | (4) Distillation under reduced pressure |
| (e) Two liquids with large difference in boiling points. | (5) Crystallisation |
Sol: (a → 5), (b → 4), (c→1), (d → 2), (e →3)
Q51. Match the terms mentioned in Column I with the terms in Column II.
| Column I | Column II |
| (a) Carbocation | (1) Cyclohexane and 1-hexene |
| (b) Nucleophile | (2) Conjugation of electrons of C – H σbond with empty p-orbital present at adjacent positively charged carbon. |
| (c) Hyperconjugation | (3) sp2 hybridised carbon with empty p-orbital |
| (d) Isomers | (4) Ethyne |
| (e) sp hybridization | (5) Species that can receive a pair of electrons |
| (f) Electrophile | (6) Species that can supply a pair of electrons |
Sol: (a → 3); (b → 6); (c→ 2); (d→ 1); (e → 4); (f → 5)
| Column I | Column II | Explanation |
| (a) Carbocation | sp2-hybridised carbon with empty p-orbital | H3C+ is carbocation. Loss of e makes its p-orbitals empty (sp2– hybridised carbon) |
| (b) Nucleophile | Species that can supply a pair of electrons | Nucleus loving, i.e., having negative charge or excess of electrons |
| (c) Hyperconjugation | Conjugation of electrons of C – H σbond with empty p-orbital present at adjacent positively charged carbon | |
| (d) Isomers | Cyclohexane and 1-hexene | Same molecular formula but different structures |
| (e) sp hybridization | Ethyne | HC ≡ CH (sp hybridization) |
| (f) Electrophile | Species that receive a pair of electrons | Electron loving, i.e., positive charge or lack of electrons |
Q52. Match Column I with Column II.
| Column I | Column II |
| (a) Dumas method | (1) AgN03 |
| (b) Kjeldahl method | (2) Silica gel |
| (c) Carius method | (3) Nitrogen gel |
| (d) Chromatography | (4) Free radicals |
| (e) Homolysis | (5) Ammonium sulphate |
Sol: (a → 3); (b → 5); (c→ 1); (d →2); (e → 4)
| Column I | Column II | Explanation |
| (a) Dumas method | Nitrogen gel | Used for N containing compounds |
| (b) Kjeldahl method | Ammonium
sulphate |
Nitrogen converts to ammonium sulphate |
| (c) Carius method | AgN03 | Compound is heated in the presence of AgN03 |
| (d) Chromatography | Silica gel | Adsorbent used is silica gel |
| (e) Homolysis ‘ | Free radicals | Free radicals are formed by homolytic fission |
NCERT Exemplar Class 11 Chemistry Solutions
- Chapter 1 Some Basic Concepts of Chemistry
- Chapter 2 Structure of Atom
- Chapter 3 Classification of Elements and Periodicity in Properties
- Chapter 4 Chemical Bonding and Molecular Structure
- Chapter 5 States of Matter
- Chapter 6 Thermodynamics
- Chapter 7 Equilibrium
- Chapter 8 Redox Reactions
- Chapter 9 Hydrogen
- Chapter 10 The s-Block Elements
- Chapter 11 The p-Block Elements
- Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
- Chapter 13 Hydrocarbons
- Chapter 14 Environmental Chemistry
NCERT Exemplar ProblemsMathsPhysicsChemistryBiology
We hope the NCERT Exemplar Class 11 Chemistry Chapter 12 Organic Chemistry: Some Basic Principles and Techniques help you. If you have any query regarding NCERT Exemplar Class 11 Chemistry Chapter 12 Organic Chemistry: Some Basic Principles and Techniques, drop a comment below and we will get back to you at the earliest.