NCERT Exemplar Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry are part of NCERT Exemplar Class 11 Chemistry. Here we have given NCERT Exemplar Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry.
NCERT Exemplar Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
Multiple Choice Questions
Single Correct Answer Type
Q1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given below. Correct reading of mass is 3.0 g. On the basis of given data, mark the correct option out of the following statements.

Results of both the students are neither accurate nor precise.
(b) Results of student A are both precise and accurate.
(c) Results of student B are neither precise nor accurate.
(d) Results of student B are both precise and accurate.
Sol:

For both the students, average value is close to the correct value. Hence, readings of both are accurate. But readings of student A are also close to each other (differ only by 0.02) and also close to the average value, hence readings of A are also precise. But readings of B are not close to each other (differ by 0.1) and hence are not precise. Thus, results of student A are both precise and accurate.
Q2. A measured temperature on Fahrenheit scale is 200°F. What will this reading be on Celsius scale?
(a) 40°C
(b) 94°C
(c) 93.3°C
(d) 30°C
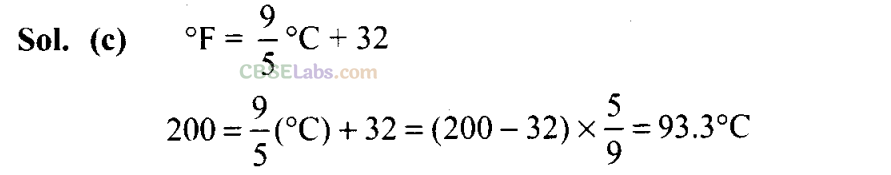
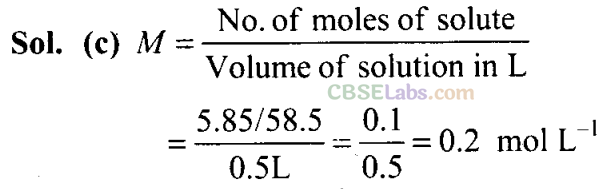
Q3. What will be the molarity of a solution, which contains 5.85 g of NaCl(s) per 500 mL?
(a) 4 mol L-1
(b) 20 mol L-1
(c) 0.2 mol L-1
(d) 2 mol L -1
Q4. If 500 mL of a 5 M solution is diluted to 1500 mL, what will be the molarity of the solution obtained?
(a) 1.5 M
(b) 1.6 M
(c) 0.017 M
(d) 1.59 M
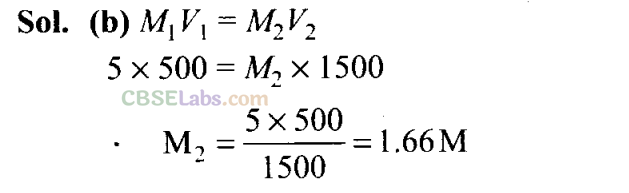
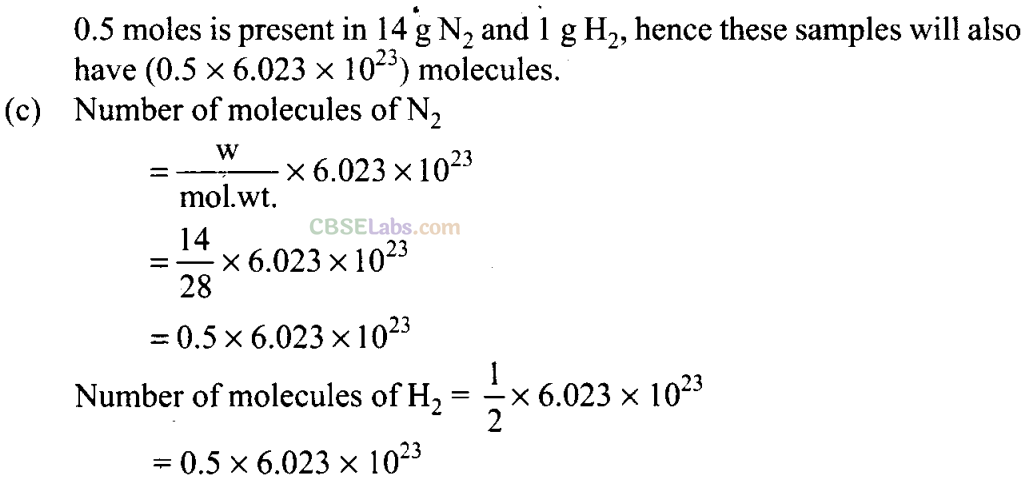
Q5. The number of atoms present in one mole of an element is equal to Avogadro number. Which of the following elements contains the greatest number of atoms?
(a) 4gHe (b) 46gNa (c) 0.40 gCa (d) 12 g He
Sol: (d) As we know that


Hence, 12 g of He contains the greatest number of atoms.
Q6. If the concentration of glucose (C6H1206) in blood is 0.9 g L-1, what will be the molarity of glucose in blood?
(a) 5 M
(b) 50 M
(c) 0.005 M
(d) 0.5 M
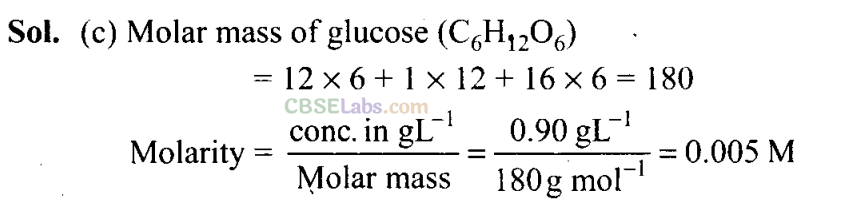
Q7. What will be the molality of the solution containing 18.25 g of HCl gas in 500 g of water?
(a) 0.1 m (b) 1 M (c) 0.5 m (d) 1 m
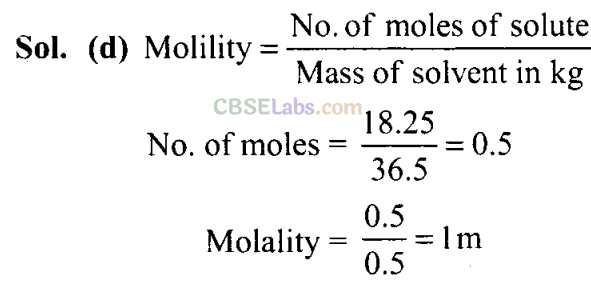
Q8. One mole of any substance contains 6.022 x 1023 atoms/molecules. Number of molecules of H2S04 present in 100 mL of 0.02 M H2S04 solution is __________
(a)12.044 x 1020 molecules
(b) 6.022 x 1023 molecules
(c) 1 x 1023 molecules
(d) 12.044 x 1023 molecules
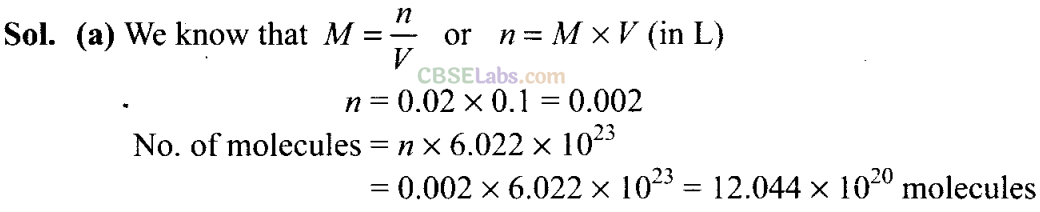
Q9. What is the mass per cent of carbon in carbon dioxide?
(a) 0.034%
(b) 27.27%
(c) 3.4%
(d) 28.7%
Sol: b) Molecular mass of C02 =1×12 + 2×16 = 44 g
1 g molecule of C02 contains 1 g atom of carbon
44 g of C02 contains C = 12 g of carbon
% of C in CO2 = 12/44 x 100 =27.27%
Hence, the mass per cent of carbon in C02 is 27.27%.
Q10. The empirical formula and molecular mass of a compound are CH20 and 180g respectively. What will be the molecular formula of the compound?
(a) C9H1809,
(b) CH20
(c) C6Hi206
(d) C2H402
Sol: (c) Empirical formula mass = 12 + 2+ 16 = 30
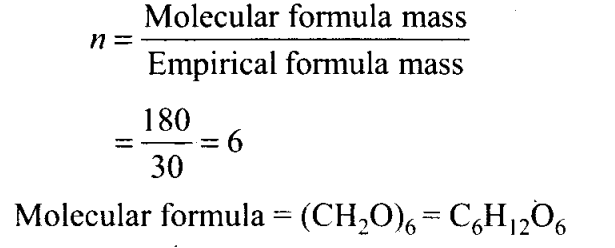
Q11. If the density of a solution is 3.12 g mL1, the mass of 1.5 mL solution in significant figures is________
(a) 4.7 g
(b) 4680 x 10-3 g
(c) 4.680 g
(d) 46.80 g
Sol: (a) Density of solution = 3.12 g mL-1
Volume of solution =1.5 mL
Mass of solution = Volume x Density
= 1.5 mL x 3.12 g mL-1
= 4.68 g = 4.7 g (up to 2 significant figures)
Q12. Which of the following statements about a compound is incorrect?
(a) A molecule of a compound has atoms of different elements.
(b) A compound cannot be separated into its constituent elements by physical methods of separation.
(c) A compound retains the physical properties of its constituent elements.
(d) The ratio of atoms of different elements in a compound is fixed.
Sol: (c) A compound does not retain the physical or chemical properties of its constituent elements.
Q13. Which of the following statements is correct about the reaction given below? 4Fe(s) + 302(g) →2Fe203(s)
(a) Total mass of iron and oxygen in reactants = total mass of iron and oxygen in product, therefore it follows law of conservation of mass.
(b) Total mass of reactants = total mass of product; therefore, law of multiple proportions is followed.
(c) Amount of Fe203 can be increased by taking any one of the reactants (iron or oxygen) in excess.
(d) Amount of Fe203 produced will decrease if the amount of any one of the reactants (iron or oxygen) is taken in excess.
Sol: (a) 4Fe + 302 —> 2Fe203 follows law of conservation of mass since mass of reactants is equal to mass of products.
Q14. Which of the following reactions is not correct according to the law of conservation of mass?
(a) 2Mg(s) + 02(g) →2MgO(s)
(b) C3H8(g) + 02(g) →C02(g) + H2O(g)
(c) P4(s) + 502(g) →P4O10(s)
(d) CH4(g) + 202(g) → C02(g) + 2H20(g)
Sol: (b) C3H8 + 02 → C02 + H20
Since the reaction is not balanced, hence, mass of reactants and products are different. It is against the law of conservation of mass.
Q15. Which of the following statements indicates that law of multiple proportions is being followed?
(a) Sample of carbon dioxide taken from any source will always have carbon and oxygen in the ratio 1:2.
(b) Carbon forms two oxides namely C02 and CO, where masses of oxygen which combine with fixed mass of carbon are in the simple ratio 2:1.
(c) When magnesium bums in oxygen, the amount of magnesium taken for the reaction is equal to the amount of magnesium in magnesium oxide formed.
(d) At constant temperature and pressure 200 mL of hydrogen will combine with 100 mL oxygen to produce 200 mL of water vapour.
Sol: (b) The element, carbon, combines with oxygen to form two compounds, namely, carbon dioxide and carbon monoxide. In C02, 12 parts by mass of carbon combine with 32 parts by mass of oxygen while in CO, 12 parts by mass of carbon combine with 16 parts by mass of oxygen.
Therefore, the masses of oxygen combine with a fixed mass of carbon (12 parts) in C02 and CO are 32 and 16 respectively. These masses of oxygen bear a simple ratio of 32 : 16 or 2 : 1 to each other.
This is an example of law of multiple proportion.
More than One Correct Answer Type
Q16. One mole of oxygen gas at STP is equal to____ .
(a) 6. 022 x 1023 molecules of oxygen
(b) 6.022 x 1023 atoms of oxygen
(c) 16 g of oxygen .
(d) 32 g of oxygen
Sol:(a,d) 1 mole of 02 gas at STP = 6.022 x 1023 molecules of 02 (Avogadro number) = 32 g of 02. Hence, 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number.
Q17. Sulphuric acid reacts with sodium hydroxide as follows:
H2S04 + 2NaOH →Na2SO4 + 2H20
When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of 0.1 M sodium hydroxide solution, the amount of sodium sulphate formed and its molarity in the solution obtained is
(a) 0.1 mol L-1
(b) 7.10 g
(c) 0.025 mol L-1
(d) 3.55 g
Sol: (b, c) Moles of H2S04 taken = 0.1 moles
Moles of NaOH taken = 0.1 moles
As H2S04 and NaOH react in ratio 1:2, so 0.1 moles of H2S04 reacts with 0.2 mole of NaOH which we don’t have.
0.1 mole of NaOH reacts with 0.05 mole of H2S04, so NaOH is Limiting reactant. Product is calculated w.r.t limiting reactant so Number of moles of Na2S04 formed will also be equal to 0.05.
Mass of Na2S04 = 0.05 x 142 = 7.1 g
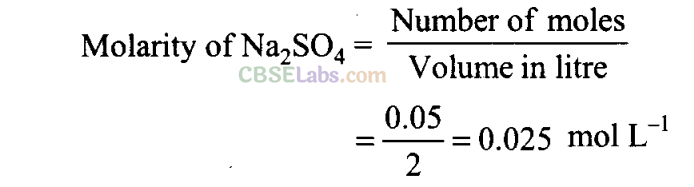
Q18. Which of the following pairs have the same number of atoms?
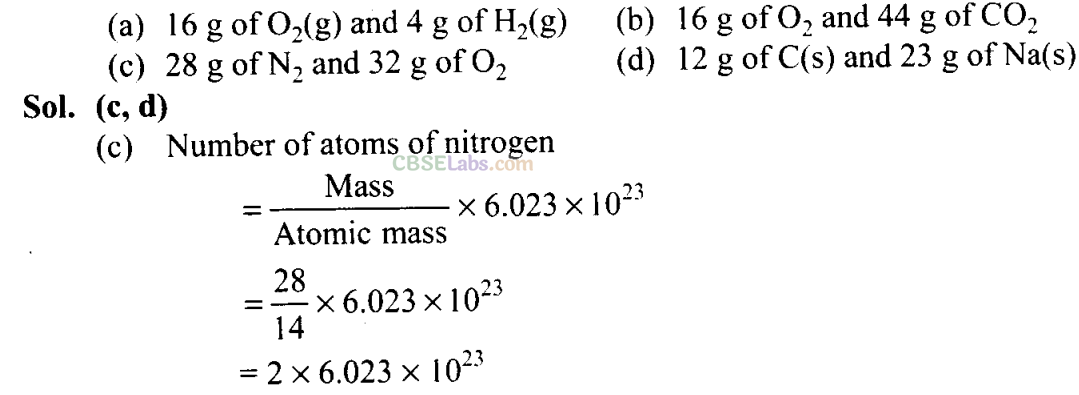
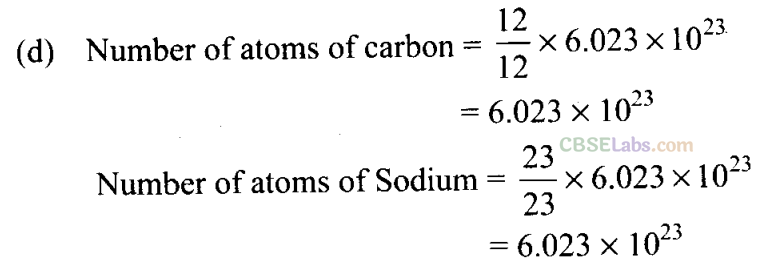
Q19. Which of the following solutions have the same concentration?
(a) 20 g of NaOH in 200 mL of solution
(b) 0.5 mol of KC1 in 200 mL of solution
(c) 40 g of NaOH in 100 mL of solution
(d) 20 g of KOH in 200 mL of solution
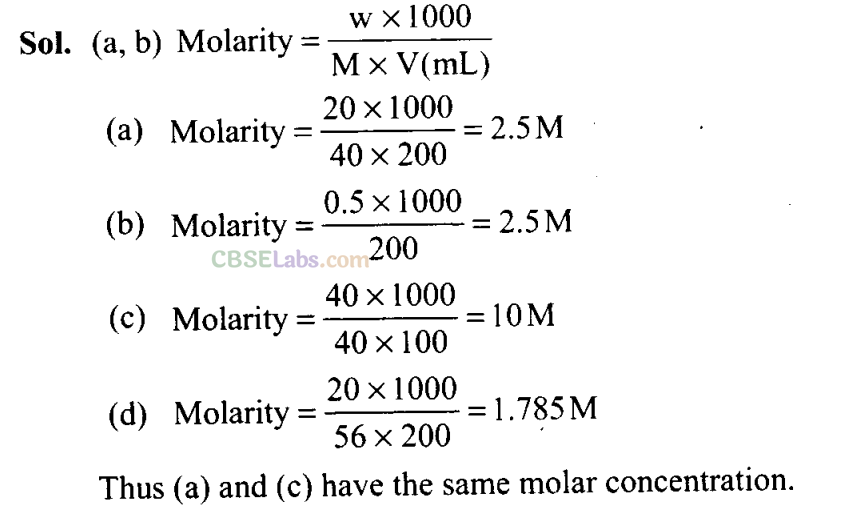
Q20. 16 g of oxygen has the same number of molecules as in
(a) 16 g of CO
(b) 28 g of N2
(c) 14g of N2
(d) 1.0 gof H2,
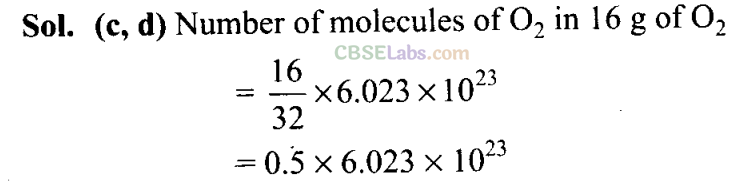
Q21. Which of the following terms are unitless?
(a) Molality
(b) Molarity
(c) Mole fraction
(d) Mass per cent
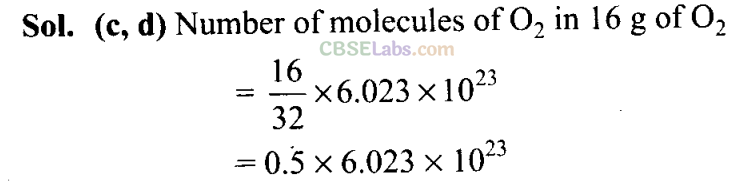
Q22. One of the statements of Dalton’s atomic theory is given below:
“Compounds are formed when atoms of different elements combine in a fixed ratio”
Which of the following laws in not related to this statement?
(a) Law of conservation of mass
(b) Law of definite proportions
(c) Law of multiple proportions
(d) Avogadro law
Sol:(a, d) The statement is related to law of definite proportions and law of multiple proportions.
Short Answer Type Questions
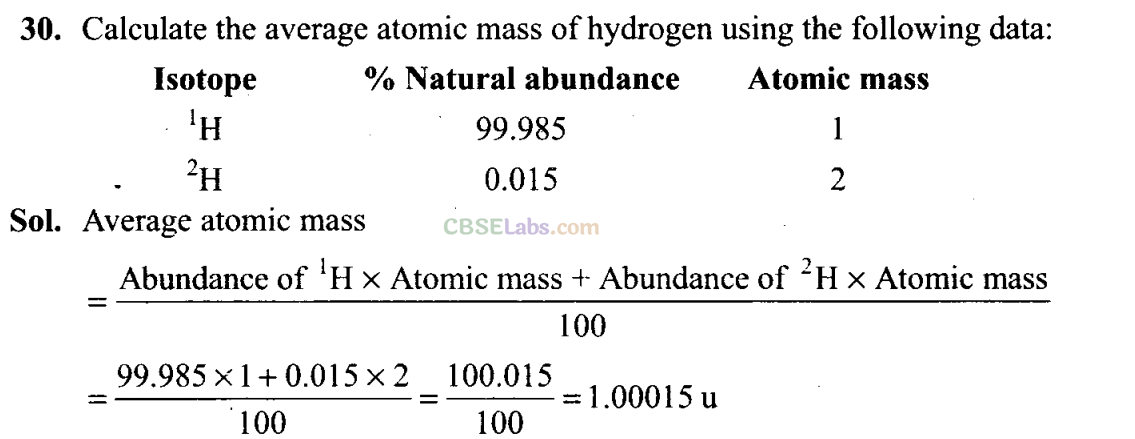
Q23. What will be the mass of one atom of C-12 ingrams?
Sol: 1 mole of carbon atoms = 12 g = 6.022 x 1023 atoms. 6.022 x 1023 atoms of carbon-12 have mass = 12 g
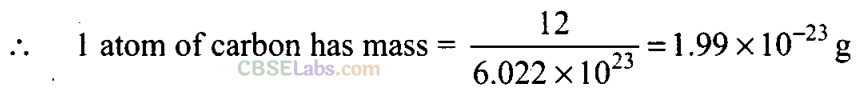
Q24. How many significant figures should be present in the answer of the following calculations?
2.5×1.25×3.5/ 2.01
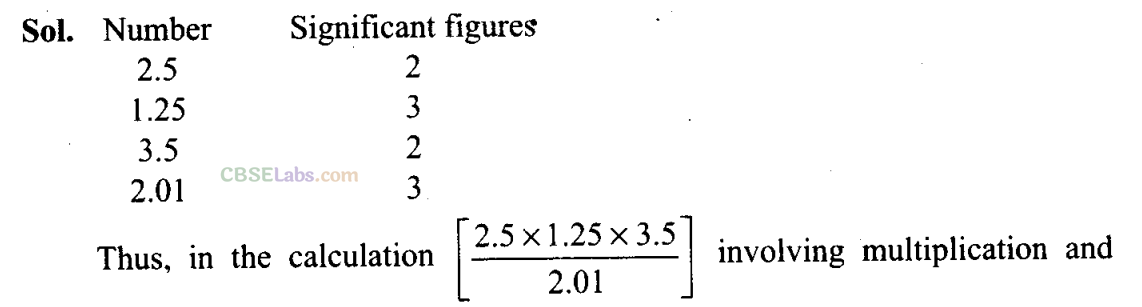
division, the number of significant figures will be 2.
Since the least number of significant figures from the given figures is 2 (in 2.5 and 3.5) the result should not have more than two significant figures.
Q25. What is the symbol for SI unit of mole? How is the mole defined?
Sol: Symbol for SI unit of mole is mol. A mole is defined as the amount of substance that contains as-many entities as there are atoms in 12 g of carbon – in C-12 isotope.
Q26. What is the difference between molality and molarity?
Sol: Molality is the number of moles of solute present in 1 kg of solvent, whereas molarity is the number of moles of solute dissolved in 1 litre of a solution. Molality is independent of temperature, whereas molarity depends on temperature.
Q27. Calculate the mass per cent of calcium, phosphorus and oxygen in calcium phosphate Ca3(P04)2.
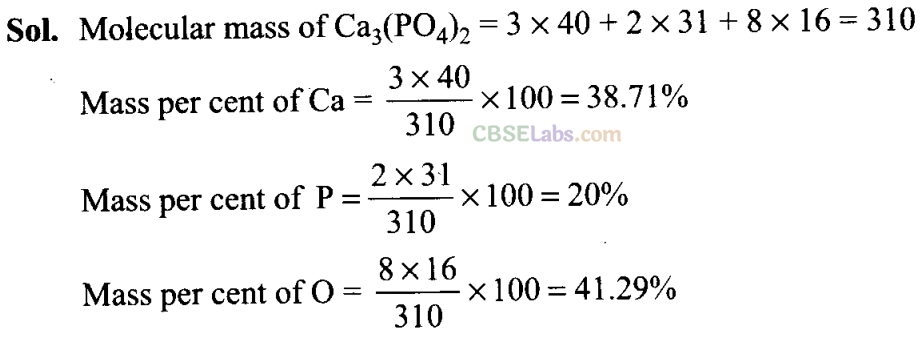
Q28. 4 L of dinitrogen reacted with 22.7 L of dioxygen and 45.4 L of nitrous oxide was formed. The reaction is given below:
2N2(g) + 02(g) —> 2N20(g)
Which law is being obeyed in this experiment? Write the statement of the law.
Sol: Gases are reacting together to form gaseous products. Ratio of volumes of gases:
N2: 02: N20 = 45.4 : 22.7 : 45.4 –
=2:1:2
Which is a simple whole number ratio. Hence the experiment proves Gay- Lussac’s law of gaseous volumes. This law states that gases combine or are produced in a chemical reaction in a simple whole number ratio by volume provided that all gases are at the same temperature and pressure.
Q29. If two elements can combine to’form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in whole number ratio.
(a) Is this statement true?
(b) If yes, according to which law?
(c) Give one example related to this law
Sol: (a) Yes, the statement is true.
(b) According to law of multiple proportions.
(c) Hydrogen and oxygen react to produce two compounds, water and hydrogen peroxide. Masses of oxygen which combine with fixed mass of hydrogen are in simple ratio.
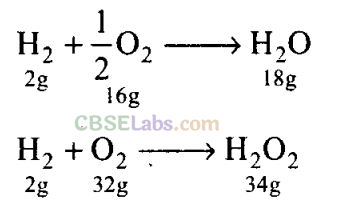
Masses of oxygen (16 and 32) which combine with fixed mass of hydrogen (2) are in the ratio of 16 : 32 or 1 : 2.
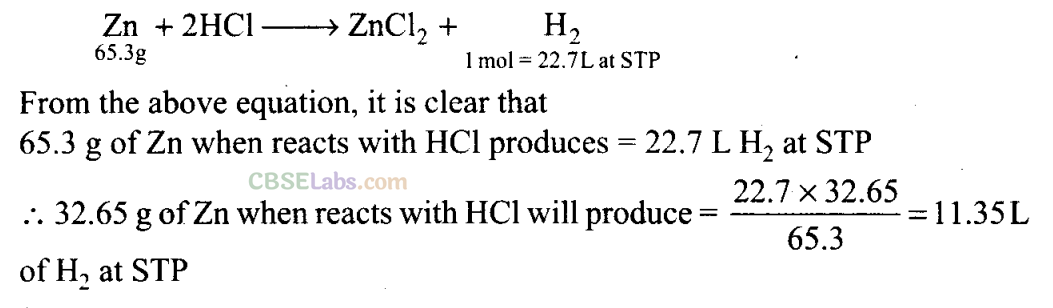
Q31. Hydrogen gas is prepared in the laboratory by reacting dilute HC1 with granulated zinc. Following reaction takes place:
Zn + 2HC1 → ZnCl2 + H2
Calculate the volume of hydrogen gas liberated at STP when 32.65 g of zinc reacts with HC1. 1 mol of a gas occupies 22.7 L volume of STP; atomic mass of Zn = 65.3 u.
Sol: Given that, mass of Zn = 32.65 g
1 mole of gas occupies = 22.7 L volume at STP Atomic mass of Zn = 65.3u
The given equation is
Q32. The density of 3 molal solution of NaOH is 1.110 g mL -1. Calculate the molarity of the solution.
Sol: 3 molal solution of NaOH means 3 moles of NaOH is dissolved in 1000 g of water.
But 3 moles of NaOH = 3 x 40 g = 120 g
120 g = 1120 g
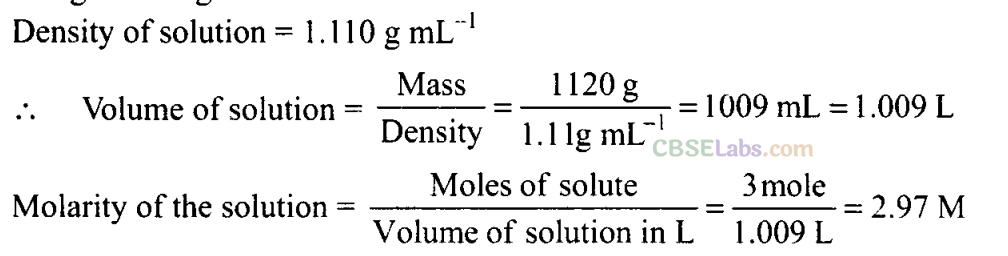
Q33. Volume of a solution changes with change in temperature, then, will the molality of the solution be affected by temperature? Give reason for your answer.
Sol: No, molality of a solution does not change with temperature since mass remains unaffected by temperature.
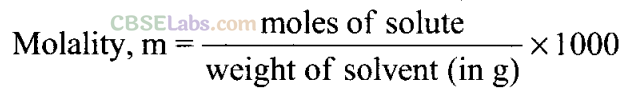
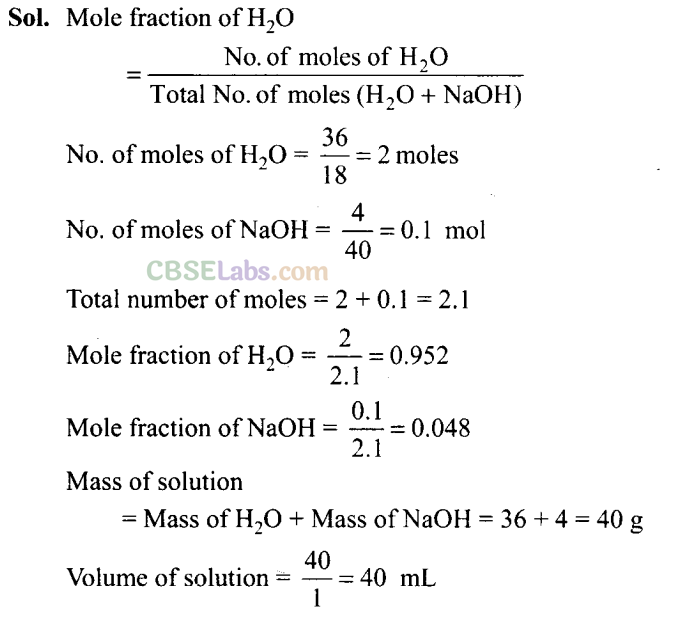
Q34. If 4 g of NaOH dissolves in 36 g of H20, calculate the mole fraction of each component in the solution. Also, determine the molarity of solution (specific gravity of solution is 1 g mL-1).
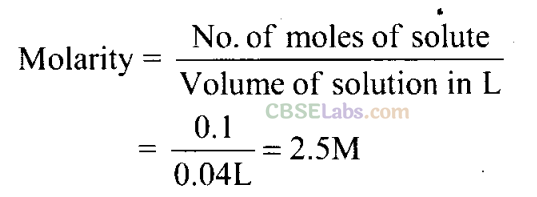
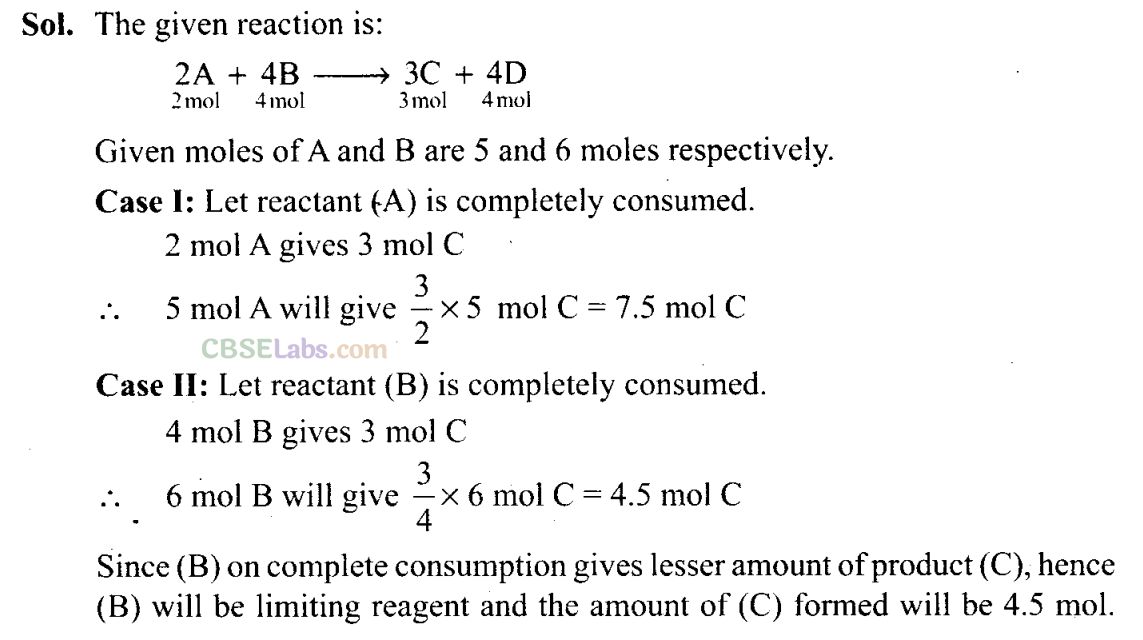
Q35. The reactant which is entirely consumed in reaction is known as limiting reagent. In the reaction 2A + 4B —> 3C + 4D, when 5 moles of A react with 6 moles of B, then
(i) which is the limiting reagent?
(ii) calculate the amount of C formed.
Matching Column Type Questions
36. Match the following :
| Column I | Column II |
| A. 88 g of C02 | a. 0.25 mol |
| B. 6.022 x 1023 molecules of H20 | b. 2 mol |
| C. 5.6 litres of 02 at STP | c. 1 mol |
| D. 96 g of 02 | d. 6.022 x 1023 molecules |
| E. 1 mole of any gas | e. 3 mol |
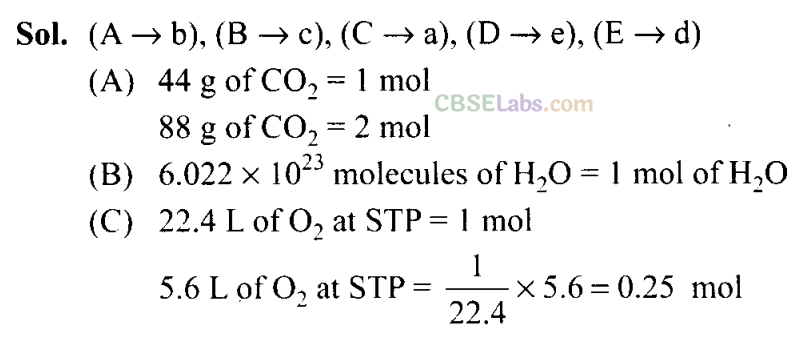
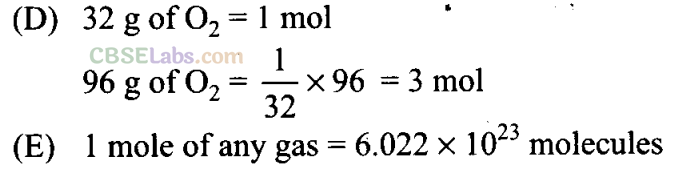
Q37. Match the following physical quantities with units
| Column I (Physical) | Column II (Unit) |
| (i) Molarity | a. g mL-1 |
| (ii) Mole fraction | b. mol |
| (iii) Mole | c. Pascal |
| (iv) Molality | d. Unitless |
| (v) Pressure | e. mol L-1 |
| (vi) Luminous intensity | f. Candela |
| (vii) Density . | g. mol kg-1 |
| (viii) Mass | h. N m-[1] |
| i. kg |
Sol: (i →e), (ii → d), (iii → b), (iv →g), (v —> c), (vi —» f), (vii → a), (viii → i)
(i) Molarity = mol L-1
(ii) Mole fraction = no units
(iii) Mole = mol
(iv) Molality = mol kg 3
(v) Pressure = Pascal or N m 2
(vi)Luminous intensity = Candela
(vii) Density = g mL-1
(viii) Mass = kg
Assertion and Reason Type Questions
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
38. Assertion (A): The empirical mass of ethene is half of its molecular mass. Reason (R): The empirical formula represents the simplest whole number ratio of various atoms present in a compound.
(a) Both A and R are true and R is the correct explanation of A.
(b) A is true but R is false
(c) A is false but R is true
(d) Both A and R are false.
Sol: (a) Empirical formula of ethene = CH2
Empirical formula mass of ethene =14 amu
= 1/2 — x molecular mass of ethene .
Empirical formula shows that ethene has (C : H):: 1 : 2
Q39. Assertion (A): One atomic mass unit is defined as one-twelfth of the mass of one carbon-12 atom.
Reason (R): Carbon-12 isotope is the most abundant isotope of carbon and has been chosen as standard.
(a) Both A and R are the true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) Both A and R are false.
Sol: (b) 1 amu = —1/12 Mass of one C12 atom
C12 isotope is considered as standard for defining the atomic and molecular mass.
Q40. Assertion (A): Significant figures for 0.200 is 3 whereas for 200 it is 1.
Reason (R): Zero at the end or right of a number are significant provided they are not on the right side of the decimal point.
(a) Both A and R are true and R is correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) Both A and R are false.
Sol: (c) Significant figures for 0.200 = 3
Significant figure for 200 = 1
Zeros at the end of a number without decimal- point, may or may not be significant depending on the accuracy of measurement.
Q41. Assertion (A): Combustion of 16 g of methane gives 18 g of water.
Reason (R): In the combustion of methane, water is one of the product.
(a) Both A and R are true but R is not the correct explanation of A.
(b) A is true but R is false
(c) A is false but R is true.
(d) Both A and R are false.
Sol: (c) CH4(g) + 202(g) → C02(g) + 2H20(g)
16 g of CH4 on complete combustion will give 36 g of water
Long Answer Type Question
Q42. A vessel contains 1.6 g of dioxygen at STP (273.15 K, 1 atm pressure). The gas is now transferred to another vessel at constant temperature, where pressure becomes half of the original pressure. Calculate
(i) volume of the new vessel.
(ii) number of molecules of dioxygen.

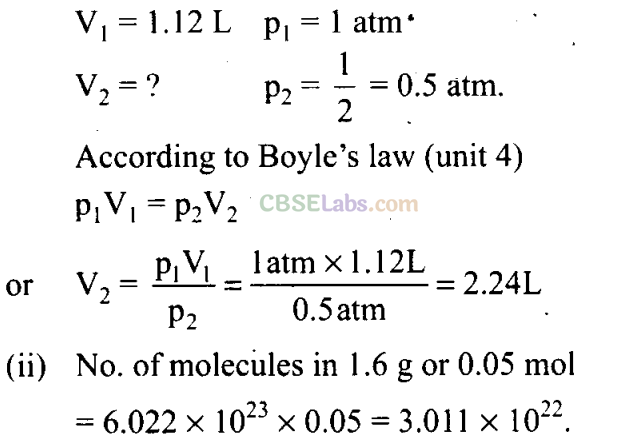
Q43. Calcium carbonate reacts with aqueous HC1 to give CaCl2 and C02 according to the reaction given below:
CaC03(s) + 2HCl(aq) → CaCl2(aq) + C02(g) + H2O(l)
What mass of CaCl2 will be formed when 250 mL of 0.76 M HC1 reacts with 1000 g of CaC03? Name the limiting reagent. Calculate the number of moles of CaCl2 formed in the reaction.
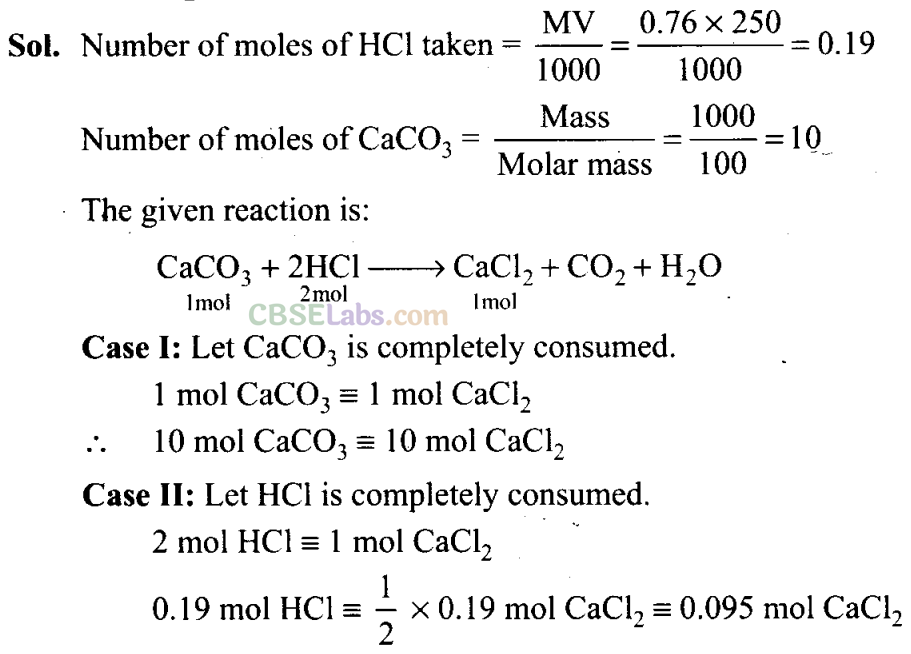
Since HCl on complete consumption gives lesser amount of product hence HC1 will be limiting reagent and the number of moles of CaCl2 formed will be 0.095 mol.
Q43. Define the law of multiple proportions. Explain it with two examples. How does this law point to the existence of atoms?
Sol: Law of multiple proportions: When two elements combine to form two or more chemical compounds, then the masses of one of the elements which combine with a fixed mass of the other, bear a simple ratio to one another, e.g., carbon combines with oxygen to form two compounds, namely, carbon dioxide and carbon monoxide.
The masses of oxygen which combine with a fixed mass of carbon in C02 and CO are 32 and 16 respectively. These masses of oxygen bear a simple ratio of 32 : 16 or 2 : 1 to each other. For example, sulphur combines with oxygen to form two compounds, namely, sulphur trioxide and sulphur dioxide.
The masses of oxygen which combine with a fixed mass of sulphur in S03 and S02 are 48 and 32 respectively. These masses of oxygen bear a simple ratio of 48 : 32 or 3 : 2 to each other. This law shows that there are constituents which combine in a definite proportion. These constituents may be atoms. Thus, the law of multiple proportions shows the existence of atoms which combine to form molecules.
Q44. A box contains some identical red coloured balls, labeled as A, each weighing 2 grams. Another box contains identical blue coloured balls, labeled as B, each weighing 5 grams. Consider the combinations AB, AB2, A2B and A2B3 and show that law of multiple proportions is applicable.
Sol:
| Combination of A and B | AB | ab2 | A,B | A2B3 |
| Mass of A (in g) | 2 | 2 | 4 | 4 |
| Mass of B (in g) | 5 | 10 | 5 | 15 |
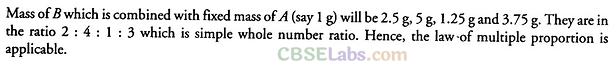
NCERT Exemplar Class 11 Chemistry Solutions
- Chapter 1 Some Basic Concepts of Chemistry
- Chapter 2 Structure of Atom
- Chapter 3 Classification of Elements and Periodicity in Properties
- Chapter 4 Chemical Bonding and Molecular Structure
- Chapter 5 States of Matter
- Chapter 6 Thermodynamics
- Chapter 7 Equilibrium
- Chapter 8 Redox Reactions
- Chapter 9 Hydrogen
- Chapter 10 The s-Block Elements
- Chapter 11 The p-Block Elements
- Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
- Chapter 13 Hydrocarbons
- Chapter 14 Environmental Chemistry
NCERT Exemplar ProblemsMathsPhysicsChemistryBiology
We hope the NCERT Exemplar Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry help you. If you have any query regarding NCERT Exemplar Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry, drop a comment below and we will get back to you at the earliest.