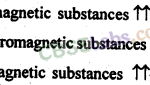Solutions Class 12 Notes Chemistry Chapter 2 1. A solution is a homogeneous mixture of two or 9. more chemically non-reacting substances. The components of a solution generally cannot be separated by filtration, settling or centrifuging. 2. A solution may be classified as solid, liquid or a gaseous solution. 3. Solubility is defined as the amount of solute in a saturated … [Read more...]
The Solid State Class 12 Notes Chemistry Chapter 1
The Solid State Class 12 Notes Chemistry Chapter 1 1. Solids are substances which have fixed shape and volume. 1’hey are characterised by rigidity, incompressibility, slow diffusion and mechanical strength. They are classified as: (a) Crystalline solids (b) Amorphous solids . 2. The crystalline solids are further classified as: (a) Metallic solids (b) Ionic solids (c) … [Read more...]
Amines Class 12 Notes Chemistry Chapter 13
Amines Class 12 Notes Chemistry Chapter 13 1. Amines are the derivatives of ammonia in which one or more hydrogen atoms have been replaced by alkyl groups. 2. Amines are classified as primary, secondary, or tertiary according as one, two or three hydrogen atoms in the ammonia molecule have been substituted by alkyl groups. 3. Preparation of amines: (i) By reduction of … [Read more...]
Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11
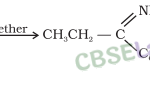
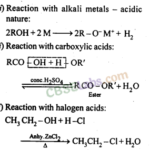
Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11 1. Alcohols and phenols may be classified as monohydric, dihydric, trihydric or polyhydric according to number of hydroxyl groups they contain one, two, three or many respectively in their molecules. 2. Primary (1°), secondary (2°) and tertiary (3°) alcohols are those in which as the OH group is attached to a … [Read more...]
Polymers Class 12 Notes Chemistry Chapter 15
Polymers Class 12 Notes Chemistry Chapter 15 1. A polymer is a large molecule of high molecular mass formed by the repetitive bonding of many small molecules called monomers. The process by which the monomers are transformed into polymers is called polymerisation. As polymers are single big size molecules, they are also called macromolecules. 2. Classification of polymers on … [Read more...]