Thermodynamics Class 11 Notes Physics Chapter 12
• The branch of physics which deals with the study of transformation of heat into other forms of energy and vice-versa is called thermodynamics.
Thermodynamics is a macroscopic science. It deals with bulk systems and does not go into the
molecular constitution of matter.
• A collection of an extremely large number of atoms or molecules confined within certain boundaries
such that it has a certain values of pressure (P), volume (V) and temperature (T) is called a ; thermodynamic system.
• Thermal Equilibrium
A thermodynamic system is in an equilibrium state if the macroscopic variables such as pressure, volume, temperature, mass composition etc. that characterise the system do not change in time. In thermal equilibrium, the temperature of the two systems are equal.
• Zeroth Law of Thermodynamics
This law identifies thermal equilibrium and introduces temperature as a tool for identifying f equilibrium. According to this law “If two systems are in thermal equilibrium with a third system then those two systems themselves are in equilibrium.”
• Heat, Work and Internal Energy
— Energy that is transferred between a system and its surroundings whenever there is temperature difference between the system and its surroundings is called heat.
— Work is said to be done if a body or a system moves through a certain distance in the direction of the applied force. It is given as
dW = PdV
where P is the pressure of the gas in the cylinder.
— If we consider a bulk system consisting of a large number of molecules, then internal energy ; of the system is the sum of the kinetic energies and potential energies of these molecules.
This energy is possessed by a system due to its molecular motion and molecular configuration. The internal energy is denoted by U.
U = Ek + Ep
where Ek and Ep represent the kinetic and potential energies of the molecules of the system.
• Internal energy of a system is a macroscopic variable and it depends only on the state of the system. Its value depends only on the given state of the system and does not depend on the path taken to arrive that state.
• First Law of Thermodynamics
The first law of thermodynamics is simply the general law of conservation of energy applied to any system. According to this law, “the total heat energy change in any system is the sum of the internal energy change and the work done.”
When a certain quantity of heat dQ is subjected to a system, a part of it is used in increasing the internal energy by dU and a part is used in performing external work dW, hence
dQ = dU + dW
• For gases, the specific heat capacity depends on the process or the conditions under which heat capacity transfer takes place. There are mainly two principal specific heat capacities for a gas. These are specific heat capacity at constant volume and specific heat capacity at constant pressure.
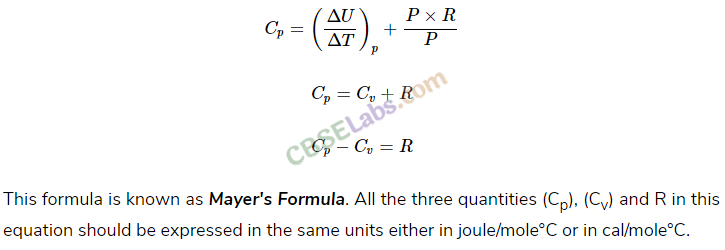
• From First Law of Thermodynamics we find a relation between two principal specific heats of an ideal gas. According to the relation
Cp-Cυ = R
Here Cp and Cυ are molar specific heats under constant pressure and constant volume condition respectively.
The specific heat capacity of a gas at constant pressure is greater than the specific heat capacity of the gas at constant volume i.e. Cp > Cυ. Reason is that when heat supplied to a gas at constant volume, no work would be done by the gas against the external pressure and all the energy is used to raise the temperature of the gas. On the other hand when the heat is supplied to the gas at constant pressure, its volume increases and the heat energy supplied to it is used to increase the temperature of the gas as well as in doing the work against the external pressure.
The difference, between the two specific heats is the thermal equivalent of the work done in expanding the gas against the external pressure.
• Expression for the Relation between Cp and Cυ
Let P, V and T be the pressure, volume and absolute temperature initially of one mole of an ideal gas.
Case (i): The heat dQ is supplied to the gas at constant volume so that the temperature increases to T + dT.
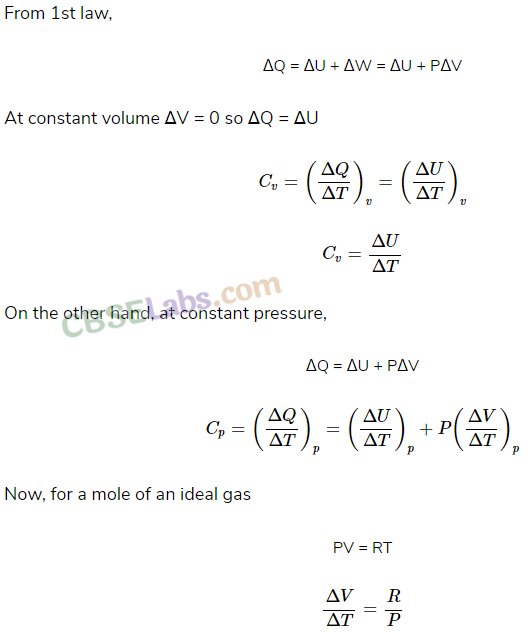
• Thermodynamic State Variables
Thermodynamic state variables of a system are the parameters which describe equilibrium states of the system. For example, equilibrium state of gas is completely specified by the values of pressure, volume, temperature, mass and composition.
• Equation of State
The equation of state represents the connection between the state variables of a system. For example, the those equation of state of an ideal/perfect gas in represented as
PV = μRT
where g is number of moles of the gas and R is gas constant for one mole of the gas.
• Thermodynamic state variables are of two kinds, extensive and intensive. Extensive variables indicate the size of the system but intensive variables do not indicate the size. Volume, mass, internal energy of a system are extensive variables but pressure, temperature and density are intensive variables.
• Thermodynamic Processes
Any process in which the thermodynamic variables of a thermodynamic system change is known as thermodynamic process.
• Quasi-Static Processes
Processes that are sufficiently slow and do not involve accelerated motion of piston and/or large temperature gradient are quasi-static processes.
In this process, the change in pressure or change in volume or change in temperature of the system is very small.
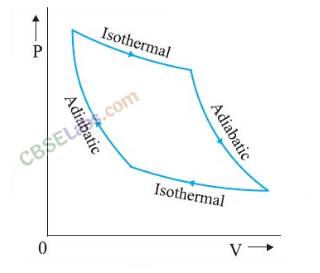
• Isothermal Process
A change in pressure and volume of a gas without any change in its temperature, is called an isothermal change. In such a change, there is a free exchange of heat between the gas and its surroundings.
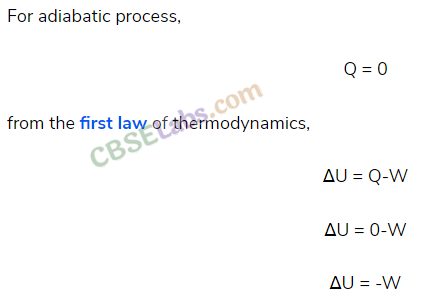
• Adiabatic Process
A process in which no exchange of heat energy takes place between the gas and the surroundings, is called an adiabatic process.
• The work done dW under isothermal change is given by
• P-V Diagram
A graph representing the variation of pressure with the variation of volume is called P-V diagram. The work done by the thermodynamic system is equal to the area under P-V diagram. It is given as
• Reversible Process
A process which can retrace so that the system passes through the same states is called a reversible process, otherwise it is irreversible.
Irreversibility arises mainly from two causes:
(i) Many processes like free expansion or an explosive chemical reaction take the system to non-equilibrium states.
(ii) Most processes involve friction, viscosity and other dissipative effects.
• Second Law of Thermodynamics
This principle which disallows certain phenomena consistent with the First law of thermodynamics is known as the second law of thermodynamics.
Following are the two statements of second law of thermodynamics.
Kelvin-Planck Statement: It is impossible to construct an engine, operating in a cycle, to extract
heat from hot body and convert it completely into work without leaving any change anywhere i.e., 100% conversion of heat into work is impossible.
Clausius Statement: It is impossible for a self acting machine, operating in a cycle, unaided by any external energy to transfer heat from a cold body to a hot body. In other words heat can not flow itself from a colder body to a hotter body.
• A heat engine is a device by which a system is made to undergo a cyclic process that results
in conversion of heat to work. Basically, a heat engine consists of: (i) a heat source, (ii) a heat sink, and (iii) a working substance.
• Carnot’s Engine. He proposed a hypothetical engine working on a cyclic/reversible process operating between two temperatures. Its efficiency is independent of the working substance and is given by, η=1-T2/T1 where T1 is the temperature of source and T2 is the temperature of sink.
• According to Carnot’s theorem: (a) working between two given temperatures T1 and T2 of the hot and cold reservoirs respectively, no engine can have efficiency more than that of Carnot’s engine, and (b) the efficiency of the Carnot engine is independent of the nature of the working substance.
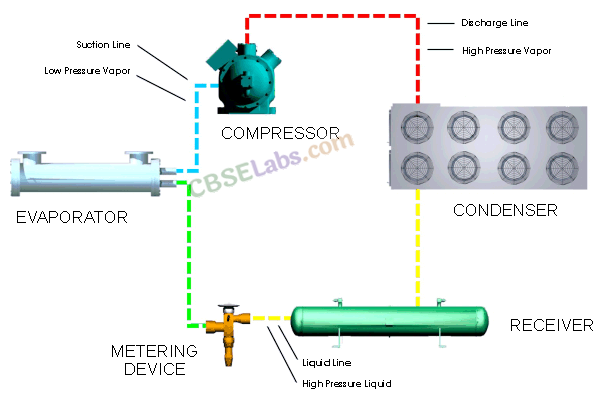
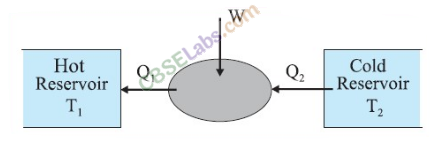
• Refrigerator
The process of removing heat from bodies colder than their surroundings is called refrigeration and the device doing so is called refrigerator.
In the refrigerator, heat is absorbed at low temperature and rejected at higher temperature with the help of external mechanical work. Thus, a refrigerator is a heat engine working backward and hence it is also called heat pump.
Refrigerator works on the reverse process of Carnot engine. By the work done on the system, heat is extracted from low temperature sink T2 and passed on to high temperature source T1. The coefficient of performance is given by
• IMPORTANT TABLES