Formulae Handbook for Class 10 Maths and Science
Lakhmir Singh Chemistry Class 10 Solutions Periodic Classification Of Elements
Lakhmir Singh Chemistry Class 10 Solutions Page No:281
Question 1:
(a) On what basis did Mendeleev arrange the elements in his periodic table ?
(B) On what basis are they arranged now ?
Solution :
(a) Mendeleev arrange the element in his periodic table on the basis of atomic masses.
(b) Now they are arranged on the basis of atomic numbers.
Question 2:
State whether the following statements are true or false :
(a) Newlands divided the elements into horizontal rows of eight elements each.
(b) According to Mendeleev’s periodic law, the properties of elements are a periodic function of their atomic numbers.
(c) The elements in a group have consecutive atomic numbers.
Solution :
(a) False
(b) False
(c) False
Lakhmir Singh Chemistry Class 10 Solutions Page No:282
Question 3:
Name the Russian chemist who said that the properties of elements are a periodic function of their atomic masses.
Solution :
Mendeleev said that the properties of elements are a periodic function of their atomic masses.
Question 4:
Rewrite the following statements after correction, if necessary :
(a) Groups have elements with consecutive atomic numbers.
(b) Periods are the horizontal rows of elements.
(c) Isotopes are the elements of the same group.
Solution :
(a) Periods have elements with consecutive atomic numbers.
(b) Correct.
(c) Correct.
Question 5:
Name the scientists who gave the following laws in the early classification of elements :
Law of octaves (b) Law of triads
Solution :
(a) Newlands.
(b) Dobereiner.
Question 6:
A, B and C are the elements of a Dobereiner’s triad. If the atomic mass of A is 7 and that of C is 39, what should be the atomic mass of B ?
Solution :
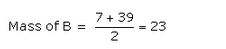
Question 7:
X and Y are the two elements having similar properties which obey Newlands’ law of octaves. How many elements are there in-between X and Y ?
Solution :
Six elements.
Question 8:
What was the Mendeleev’s basis for the classification of elements ?
Solution :
Mendeleev’s basis for the classification of elements was the atomic mass of elements.
Question 9:
In the classification of the then known elements, Mendeleev was guided by two factors. What are those two factors ?
Solution :
Mendeleev was guided by two factors:
(i) Increasing atomic masses.
(ii) Grouping together of elements having similar properties.
Question 10:
Name two elements whose properties were predicted on the basis of their positions in Mendeleev’s periodic table.
Solution :
Gallium and Scandium.
Question 11:
The three elements predicted by Mendeleev from the gaps in his periodic table were known as eka-boron, eka-aluminium and eka-silicon. What names were given to these elements when they were discovered later on ?
Solution :
Scandium, Gallium, Germanium.
Question 12:
Name two elements whose properties were predicted on the basis of their positions in Mendeleev’s periodic table.
Solution :
Eka-Aluminium (gallium) and Eka-Silicon (germanium).
Question 13:
State one example of a Dobereiner’s triad, showing in it that the atomic mass of middle element is half-way between those of the other two.
Solution :
The elements lithium, sodium and potassium form a Dobereiner’s triad. Lithium is the first element of this triad, sodium is the middle element whereas potassium is the third element of the triad. Sodium (middle element) has atomic mass 23.
According to Dobereiner,
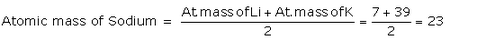
Question 14:
Which group of elements could be placed in Mendeleev’s periodic table later on, without disturbing the original order ? Give reason.
Solution :
Noble gases. Since they are chemically unreactive, so they got a place in the periodic table in the form of a separate group and hence did not disturb the original order of Mendeleev’s periodic table
Question 15:
Fill in the following blanks with suitable words :
(a) The basis for modern periodic table is………
(b) The horizontal rows in a periodic table are called……….
(c) Group 1 elements are called………
(d) Group 17 elements are known as………
(e) Group 18 elements are called…….
(f) According to Newlands’ classification of elements, the properties of sulphur are similar to those of oxygen
because sulphur is the………. element starting from oxygen.
Solution :
(a) Atomic number .
(b) Periods.
(c) Alkali metals .
(d) Halogens .
(e) Noble Gases .
(f) Eighth.
Question 16:
(a) What is meant by (i) a group, and (ii) a period, in a periodic table ?
(b) How many periods and groups are there in the long form of periodic table ?
(c) Give two examples each of (i) group 1 elements (ii) group 17 elements (iii) group 18 elements.
Solution :
(a)(i) The vertical columns in a periodic table are called groups.
(ii) The horizontal rows of elements in a periodic table are called periods.
(b) There are seven periods and eighteen groups in the long form of periodic table.
(c)(i) Group 1: Lithium and Sodium.
(ii) Group 17: Fluorine and Chlorine.
(iii) Group 18: Neon and Argon.
Question 17:
(a) In the modern periodic table, which are the metals among the first ten elements ?
(b) What is the significance of atomic number in the modern classification of elements ? Explain with the help of an example.
Solution :
(a) Lithium, Beryllium are metals.
(b) The real significance of atomic number in the modern periodic classification is that it relates the periodicity in the properties of elements to the periodicity in their electronic configurations.
Example: The atomic number increases from 3 in lithium to 11 in sodium, there is a repetition of electronic configuration from 2,1 to 2,8,1 (both having 1 valence electron).
Question 18:
(a) How were the positions of isotopes of an element decided in the modern periodic table ?
(b) How were the positions of cobalt and nickel resolved in the modern periodic table ?
(c) Where should hydrogen be placed in the modern periodic table ? Give reason for your answer.
Solution :
(a) Position of isotopes: All the isotopes of an element have the same number of protons, so their atomic number is also the same. Since, all the isotopes of an element have the same atomic number; they can be put at one place in the same group of the periodic table.
(b) Position of Cobalt and Nickel: The atomic number of cobalt is 27 and that of nickel is 28. According to modern periodic law, the elements are arranged in order of increasing atomic numbers. So, cobalt with lower atomic number (27) should come first and nickel with higher atomic number (28) should come later, even if their atomic masses are in the wrong order.
(c) Position of hydrogen: Hydrogen has been placed at the top of group 1, above the alkali metals in the modern periodic table because the electronic configuration of hydrogen is similar to those of alkali metals. Both, have 1 valence electron each.
Question 19:
(a) On which side of the periodic table will you find metals ?
(b) On which side of the periodic table will you find non-metals ?
(c) What is the name of those elements which divide metals and non-metals in the periodic table ?
Solution :
(a) Left side.
(b) Right side.
(c) Metalloids.
Lakhmir Singh Chemistry Class 10 Solutions Page No:283
Question 20:
(a) Name three elements that have a single electron in their outermost shells.
(b) Name two elements that have two electrons in their outermost shells.
(c) Name three elements with completely filled outermost shells.
Solution :
(a) Lithium, Sodium, Potassium .
(b) Magnesium, Calcium .
(c) Helium, Neon, Argon .
Question 21:
What is Debereiner’s law of triads ? Explain with the help of one example of a Dobereiner’s triad.
Solution :
Dobereiner’s law of triads: When elements are arranged in order of increasing atomic masses, groups of three elements (triads), having similar chemical properties are obtained. The atomic mass of the middle elements of the triad being equal to the arithmetic mean of the atomic masses of the other two elements.
For example: Alkali metal group ( Dobereiner’s triad) : Lithium is the 1st element, sodium is the middle element whereas potassium is the 3rd element of the triad.
Question 22:
What is Newlands’ law of octaves ? Explain with an example.
Solution :
According to the Newlands’ law of octaves, when elements are arranged in the order of increasing atomic masses, the properties of the eighth element (starting from a given element) are a repetition of the properties of the first element.
For example: If we start with lithium as the first element, we find that the eighth element from it is sodium having the similar properties to lithium.
Question 23:
(a) Did Dobereiner’s triads also exist in the columns of Newlands’ law of octaves ? Explain your answer.
(b) What were the limitations of Dobereiner’s classification of elements ?
(c) What were the limitations of Newlands’ law of octaves ?
Solution :
(a)Yes,Dobereiners triads also exist in the columns of Newlands’ Octaves.
Consider the elements lithium (Li), sodium (Na) and potassiu m (K) which are present in the second column of Newlands’ classification of elements. Now, if we start with lithium as the 1 st element, then the 8 th element from it is sodium, and according to Newlands’ law of octaves, the properties of 8 th element , sodium should be similar to thos e of the 1 st element, lithium. Again, if we take sodium as the 1 st el ement , then the 8 th element from it is potassium, and according to Newlands ‘ law of octaves, the properties of 8 th element, potassium should be similar to those of the 1 st element, sodium. This means that according to Newlands’ law of octaves, the elements lithium, sodium and potassium should have similar chemical properties. We also know that lithium, sodium and potassium form a Dobereiner’s triad having similar chemical properties. From this, we conclude that Dobereiners triads also exist in the columns of Newlands Octaves.
(b) The main limitation of Dobereiner’s classification of elements was that it failed to arrange all the then known elements in the form of triads of elements having similar chemical properties. Dobereiner could identify only three triads from the elements known at that time. So, his classification of elements was not much successful. Another limitation was that Dobereiner failed to explain the relation between atomic masses of elements and their chemical properties.
(c) Newlands’ law of octaves for the classification of elements had the following limitations:
( i ) Newlands’ law of octaves was applicable to the classification of elements up to calcium only. After calcium, every eighth element did not possess the properties similar to that of the first element. Thus, this law worked well with lighter elements only.
(ii) Newlands assumed that only 56 elements existed in nature and no more elements would be discovered in the future. But later on, several new elements were discovered whose properties did not fit into Newlands’ law of octaves.
(iii) In order to fit elements into his table, Newlands put even two elements together in one slot and that too in the column of unlike elements having very different properties. For example, the two elements cobalt (Co) and nickel (Ni) were put together in just one slot and that too in the column of elements like fluorine, chlorine and bromine which have very different properties from these elements.
Question 24:
(a) State the periodic law on which Mendeleev’s periodic table was based. Why and how was this periodic law changed ?
(b) Explain why, the noble gases are placed in a separate group.
Solution :
(a) According to Mendeleev’s periodic law: The properties of elements are a periodic function of their atomic masses. It was the discovery of atomic number which led to a change in Mendeleev’s periodic law which was based on atomic mass.
(b) The noble gases are placed in a separate group because they are chemically very inert or unreactive (having completely filled outermost electron shells).
Question 25:
(a) State the merits of Mendeleev’s classification of elements.
(b) Describe two anomalies of Mendeleev’s periodic classification of elements.
Solution :
(a) Merits of Mendeleev’s classification of elements:
(i) Mendeleev’s periodic law predicted the existence of some elements that had not been discovered at that time.
(ii) Mendeleev’s periodic table could predict the properties of several elements on the basis of their positions in the periodic table.
(iii) It could accommodate noble gases when they were discovered.
(b) Anomalies of Mendeleev’s classification of elements:
(i) The position of isotopes could not be explained: If the elements are arranged according to atomic masses, the isotopes should be placed in different groups of the periodic table. But, the isotopes were not given separate places in Mendeleev’s periodic table. They were placed at the same place in the table. This placing of the isotopes at same place could not be explained by Mendeleev’s periodic law.
(ii) Wrong order of atomic masses of some elements could not be explained: In Mendeleev’s periodic table, when certain elements were put in their correct group on the basis of their chemical properties, it was found that the element with higher atomic mass comes first and the element with lower atomic mass comes later. Mendeleev’s periodic law could not explain this abnormal situation of wrong order of atomic masses.
Question 26:
(a) How do the properties of eka-aluminium element predicted by Mendeleev compare with the actual properties of gallium element ? Explain your answer.
(b) What names were given by Mendeleev to the then undiscovered elements (i) scandium (ii) gallium, and (iii) germanium ?
Solution :
(a) Eka-aluminium and gallium are the two names of the same element as Eka -Aluminum has almost exactly the same properties as the actual properties of the gallium element. The properties: atomic mass, density, melting point, formula of chloride and formula of oxide are almost the same.
(b) (i) Eka boron.
(ii) Eka aluminum.
(iii) Eka -silicon.
Question 27:
(a) Why do we classify elements ?
(b) What were the two criteria used by Mendeleev to classify the elements in his periodic table ?
(c) Why did Mendeleev leave some gaps in his periodic table ?
(d) In Mendeleev’s periodic table, why was there no mention of noble gases like helium, neon and argon ?
(e) Would you place the two isotopes of chlorine, Cl-35 and Cl-37 in different slots because of their different atomic masses or in the same slot because their chemical properties are the same ? Justify your answer .
Solution :
(a) The elements are classified into groups so that the elements with similar properties fall in the same group and hence the study of a large number of elements is reduced to the study of a few group of elements.
(b)(i) Increasing atomic masses
(ii) Grouping together of elements having similar properties.
(c) In order to make sure that the elements having similar properties fell in the same vertical column or group, Mendeleev left some gaps in his periodic table.
(d) Out of eight groups in the original periodic table of Mendeleev, first seven groups are of normal elements and eighth group is of transition elements. Noble gases were not known at that time. So, there was no group of noble gases in Mendeleev’s table.
(e) The isotopes of chlorine, Cl-35 and Cl-37 are placed in the same slot because they have similar chemical properties and same atomic number.
Question 28:
(a) State Mendeleev’s periodic law.
(b) What chemical properties of elements were used by Mendeleev in creating his periodic table ?
(c) State any three limitations of Mendeleev’s classification of elements.
(d) Besides gallium, which two other elements have since been discovered for which Mendeleev had left gaps in his periodic table ?
(e) Which group of elements was missing from Mendeleev’s original periodic table ?
Solution :
(a) Mendeleev’s periodic law: The properties of elements are a periodic function of their atomic masses. It was the discovery of atomic number which led to a change in Mendeleev’s periodic law which was based on atomic mass.
(b) The elements having similar chemical properties form oxides and hydrides having similar formulae. Mendeleev used these properties for creating his periodic table.
(c) Limitations of Mendeleevs classification of elements:
(i) The position of isotopes could not be explained.
(ii) Wrong order of atomic masses of some elements could not be explained.
(iii) A correct position could not be assigned to Hydrogen in the periodic table.
(d) Silicon and Germanium.
(e) Noble gases were missing from Mendeleev’s original periodic table.
Question 29:
(a) State modern periodic law.
(b) How does the electronic configuration of the atom of an element relate to its position in the modern periodic table ?
(c) How could the modern periodic law remove various anomalies of Mendeleev’s periodic table ? Explain with examples.
(d) Is it possible to have an element having atomic number 1.5 placed between hydrogen and helium ?
(e) Name the scientist who prepared modern periodic table.
Solution :
(a) The modern periodic law states that the properties of elements are a periodic fu nction of their atomic numbers.
(b) When elements are arranged according to increasing atomic numbers, there is a periodicity in the electronic configurations of the elements. The elements in a period have consecutive atomic numbers. The elements having same number of valence electrons in their atoms are placed in a group. All the elements in a group have similar electronic configurations and show similar properties.
(c) When the elements are arranged according to their atomic numbers on the basis of modern periodic law, then all the anomalies (or defects) of Mendeleev’s classification disappear. This is discussed below:
(i) Explanation for the Position of Isotopes: All the isotopes of an element have the same number of protons, so their atomic number is also the same. Since all the isotopes of an element have the same atomic number, they can be put at one place in the same group of the periodic table. For example, both the isotopes of chlorine, Cl-35 and Cl-37, have the same atomic number of 17, so both of them can be put at one place in the same group of the periodic table.
(ii) Explanation for the Position of Cobalt and Nickel: The atomic number of cobalt is 27 and that of nickel is 28. Now, according to modern periodic law, the elements are arranged in the order of increasing atomic numbers. So, cobalt with lower atomic number (27) should come first and nickel with higher atomic number (28) should come later, even if their atomic masses are in the wrong order.
(iii) Explanation for the Position of Hydrogen: Hydrogen element has been placed at the top of group 1, above the alkali metals because the electronic configuration of hydrogen is similar to those of alkali metals. Both, hydrogen as well as alkali metals have 1 valence electron each.
(d) Atomic number is always a simple whole number. It can either be 1 or 2. There can be no element with atomic number 1.5.
(e) The modern periodic table was prepared by Bohr .
Lakhmir Singh Chemistry Class 10 Solutions Page No:284
Question 42:
The atomic masses of three elements X, Y and Z having similar chemical properties are 7, 23 and 39 respectively.
(a) Calculate the average atomic mass of elements X and Z.
(b) How does the average atomic mass of elements X and Z compare with the atomic mass of element Y ?
(c) Which law of classification of elements is illustrated by this example ?
(d) What could the elements X, Y and Z be ?
(e) Give another example of a set of elements which can be classified according to this law.
Solution :
(a)
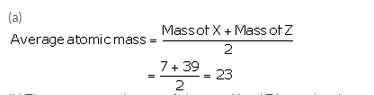
(b) The average atomic mass of elements X and Z is equal to the atomic mass of element Y .
(c) Dobereiner’s law of triads.
(d) X is lithium, Y is sodium and Z is potassium .
(e) Chlorine, Bromine, Iodine .
Question 43:
In the following set of elements, one element does not belong to the set. Select this element and explain why it does not belong :
Calcium, Magnesium, Sodium, Beryllium
Solution :
Sodium does not belong to the set. This is because all other elements belong to group 2 but sodium belongs to group 1 .
Question 44:
In the following set of elements, one element does not belong to the set. Select this element and state why it does not belong :
Oxygen, Nitrogen, Carbon, Chlorine, Fluorine
Solution :
Chlorine does not belong to the set. This is because all other elements belong to 2nd period whereas chlorine belongs to 3rd period.
Question 45:
Can the following groups of elements be classified as Dobereiner’s triads ?
(a) Na, Si, Cl (b) Be, Mg, Ca
Give reason for your answer.
(Atomic masses : Be 9 ; Na 23 ; Mg 24 ; Si 28 ; Cl 35.5 ; Ca 40)
Solution :
(a) No. This is because the elements Na, Si and Cl do not have similar properties even though the atomic mass of middle element Si is almost equal to the average atomic mass of first element Na and third element Cl.
(b) Yes. This is because the elements Be, Mg and Ca have similar properties and the atomic mass of middle element Mg is almost equal to the average atomic mass of first element Be and third element Ca
Question 46:
Consider the following elements :
Na, Ca, Al, K, Mg, Li
(a) Which of these elements belong to the same period of the periodic table ?
(b) Which of these elements belong to the same group of the periodic table ?
Solution :
(a) Same period (Third period): Na, Mg, Al.
(b) Same group (First group): Li, Na, K .
Question 47:
Which element has :
(a) two shells, both of which are completely filled with electrons ?
(b) the electronic configuration 2, 8, 2 ?
(c) a total of three shells, with four electrons in its valence shell ?
(d) a total of two shells, with three electrons in its valence shell ?
(e) twice as many electrons in its second shell as its first shell ?
Solution :
(a) Neon (2, 8).
(b) Magnesium .
(c) Silicon (2, 8, 4).
(d) Boron (2, 3) .
(e) Carbon (2, 4) .
Lakhmir Singh Chemistry Class 10 Solutions Page No:285
Question 48:
Consider the following elements :
Ca, Cl, Na, I, Li, Ba, Sr, K, Br
Separate these elements into three groups (families) of similar properties. State one property in each case on the basis of which you have made your choice.
Solution :
Li,Na,K : All these elements ar e metals having a valency of 1.
Ca,Sr,Ba: All these elements are metals having a valency of 2.
Cl,Br,I : All these elements are halogens.
Question 49:
Mendeleev predicted the existence of certain elements not known at that time and named two of them as eka-aluminium, and eka-silicon.
(a) Name the element which has taken the place of (i)eka-aluminium, and (ii)eka-silicon
(b) Mention the period/periods of these elements in the modern periodic table.
(c) Write the group/groups of these elements in the modern periodic table.
(d) Classify these elements as metals, non-metals or metalloids.
(e) How many valence electrons are present in the atoms of each of these elements ?
Solution :
(a) (i) Gallium (ii) Germanium.
(b) 4th period .
(c) Gallium: 13th group; Germanium: 14th group .
(d) Gallium: Metal; Germanium: Metalloid .
(e) Gallium: 3 ; Germanium: 4 .
Question 50:
A part of the early classification of elements has been given below :
H Li Be B C N O
F Na Mg A1 Si P S
(a) Which law of classification of elements is illustrated by the above arrangement of elements ?
(b) Name the scientist who proposed such a classification of elements.
(c) Why is such a classification of elements compared with a characteristic of musical scale ?
(d) State one limitation of this classification of elements.
Solution :
(a) Newlands’ law of octaves .
(b) Newlands .
(c) This classification of elements is compared with a characteristic of musical scale because in this classification, the repetition in the properties of elements is just like the repetition of eighth note in an octave of music .
(d) This classification of elements could be applied only up to the element calcium and not beyond that .
Lakhmir Singh Chemistry Class 10 Solutions Page No:302
Question 1:
Given alongside is a part of the periodic table :
As we move horizontally from left to right :
(i) What happens to the metallic character of the elements ?
(ii) What happens to the atomic size ?

Solution :
(i) Metallic character decreases.
(ii) Atomic size decreases .
Question 2:
How would the tendency to gain electrons change on moving from left to right in a period of the periodic table ?
Solution :
On moving from left to right in a period, the tendency of atoms to gain electrons increases.
Question 3:
How would the tendency to lose electrons change as we go from left to right across a period of the periodic table ?
Solution :
On moving from left to right in a period, the tendency of atoms to lose electrons decreases
Lakhmir Singh Chemistry Class 10 Solutions Page No:303
Question 4:
(a) How does the chemical reactivity of alkali metals vary on going down in group 1 of the periodic table ?
(b) How does the chemical reactivity of the halogens vary on going down in group 17 of the periodic table ?
Solution :
(a) In group 1 of alkali metals, the chemical reactivity increases from lithium to francium.
(b) In group 17 of halogen elements, the chemical reactivity decreases from fluorine to iodine.
Question 5:
What property do all elements in the same column of the periodic table as boron have in common ?
Solution :
All elements in the same column of the periodic table as boron have 3 valence electrons.
Question 6:
What property do all the elements in the same group of the periodic table as fluorine have in common ?
Solution :
The element fluorine is in group 17 o f the periodic table and has a valency of 1. So, all the elements in the same group of periodic table as fluorine will have a valency of 1.
Question 7:
(a) What is the number of valence electrons in the atoms of first element in a period ?
(b) What is the usual number of valence electrons in the atoms of the last element in a period ?
Solution :
(a) 1
(b) 8
Question 8:
State whether the following statement is true or false :
On going down in a group of the periodic table, the number of valence electrons increases.
Solution :
False
Question 9:
What is the major characteristic of the first elements in the periods of the periodic table ? What is the general name of such elements ?
Solution :
The first elements in the periods of the periodic table have 1 valence electron. Such elements are called alkali metals.
Question 10:
How do the atomic radii of elements change as we go from left to right in a period of the periodic table ?
Solution :
On moving from left to right in a period, the atomic size decreases.
Question 11:
What happens to the metallic character of the elements as we go down in a group of the periodic table ?
Solution :
On going down in a group of the periodic table, the metallic character of elements increases.
Question 12:
How does the number of valence electrons vary on moving from left to right :
in the first period of the periodic table ? (if) in the second period of the periodic table ?
Solution :
(i) The number of valence electrons increases from 1 to 2 in the 1st period of the periodic table.
(ii) The valence electrons increase from 1 to 8 in the 2nd period of the periodic table.
Question 13:
How does the valency of elements change on moving from left to right in the third period of the periodic table ?
Solution :
The valency of elements increases from 1 to 4 and then decreases to zero in the 3rd period.
Question 14:
How does the valency of elements vary in going down a group of the periodic table ?
Solution :
All the elements in a group have the same valency while going down the group.
Question 15:
Name the element which is in :
(a) first group and third period. (b) seventeenth group and second period.
Solution :
(a) Sodium.
(b) Fluorine .
Question 16:
How do electronic configurations of elements change in second period of periodic table with increase in atomic numbers ?
Solution :
2, 1 ; 2, 2 ; 2,3 ; 2,4 ; 2, 5 ; 2, 6 ; 2, 7 ; 2, 8 .
Question 17:
Arrange the following elements in increasing order of their atomic radii :
Li, Be, F, N
Solution :
F < N < Be < Li .
Question 18:
Arrange the following elements in the increasing order of their metallic character :
Mg, Ca, K, Ga
Solution :
Ga < Mg < Ca < K .
Question 19:
Rewrite the following statements after correction, if necessary :
(i) Elements in the same period have equal valency
(ii) The metallic character of elements in a period increases gradually on moving from left to right.
Solution :
(i) Elements in th e same group have equal valency.
(ii) The metallic character of elements in a period decreases gradually on moving from left to right
Question 20:
Fill in the blanks in the following statements :
(a) The horizontal rows in a periodic table are called……….
(b) In going across a period (right to left)in periodic table, the atomic size of the atom………
(c) On moving from right to leftin the second period, the number of valence electrons……….
(d) On going down in a group in the periodic table, the metallic character of elements………..
(e) The tendency to gain an electron…….. on moving down in a group of the periodic table.
Solution :
(a) Periods
(b) Increases
(c) Decreases
(d) Increases
(e) Decreases
Question 21:
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the periodic table. Write the electronic configurations of these two elements. Which of these will be more electronegative ? Why ?
Solution :
N (2 ,5 ) ; P (2, 8, 5) ; Nitrogen will be more electronegative because its atom has small size due to which the attraction of its nucleus for the incoming electron is more
Question 22:
An element X belongs to group 2 and another element Y belongs to group 15 of the periodic table :
(a) What is the number of valence electrons in X ? (b) What is the valency of X ?
(c) What is the number of valence electrons in Y ? (d) What is the valency of Y ?
Explain how you have arrived at your answers.
Solution :
(a) 2.
For groups 1 and 2, the number of valence electrons is equal to the group number.
(b) 2 .
Valency is determined by the number of valence electrons present in the atom of the element.
(c) 5 .
For groups 13 to 18, the number of valence electrons is equal to (group no. – 10).
(d) 3 .
The number of electrons lost or gained by one atom of an element to achieve the nearest inert gas configuration, gives us the valency.
Question 23:
(a) What is a period in a periodic table ? How do atomic structures (electron arrangements) change in a
period with increase in atomic numbers from left to right ?
(b) How do the following change on going from left to right in a period of the periodic table ?
(i) Chemical reactivity of elements (ii) Nature of oxides of elements
Give examples in support of your answer.
Solution :
(a) The horizontal rows of elements in a periodic table are called periods. As we move from left to right in a period, the atomic number of elements increases which means that the no. of protons and electrons in the atom increases. Due to large positive charge on the nucleus, the electrons are pulled in more close to the nucleus and the size of the atom decreases.
(b) (i) On moving from left to right in a period, the chemical reactivity of elements first decreases and then increases.
Example: In the 3rd period of elements, sodium is a very reactive element, magnesium is less reactive whereas aluminium is still less reactive. Silicon is the least reactive in the third period. Now, phosphorus is quite reactive, sulphur is still more reactive whereas chlorine is very reactive.
(ii) On moving from left to right in a period, the basic nature of oxides decreases and the acidic nature of oxides increases.
Example: In the 3rd period of the periodic table, sodium oxide is highly basic in nature and magnesium oxide is comparatively less basic. The aluminium and silicon oxides are amphoteric in nature. Phosphorus oxides are acidic, sulphur oxides are more acidic whereas chlorine oxides are highly acidic in nature.
Question 24:
(a) How does the size of atoms (atomic size) generally vary in going from left to right in a period of the
periodic table ? Why does it vary this way ?
(b) What happens to the metallic character of the elements as we move from left to right in a period of the periodic table ?
Solution :
(a) On moving from left to right in a period of the periodic table, the atomic size decreases. As we move from left to right in a period, the atomic number of elements increases which means that the no. of protons and electrons in the atoms increases. Due to large positive charge on the nucleus, the electrons are pulled in more close to the nucleus and the size of atom decreases.
(b) On moving from left to right in a period, the metallic character of elements decreases.
Lakhmir Singh Chemistry Class 10 Solutions Page No:304
Question 25:
(a) Explain why :
All the elements of a group have similar chemical properties.
All the elements of a period have different chemical properties.
The atomic radii of three elements X, Y and Z of a period of the periodic table are 186 pm; 104 pm and 143 pm respectively. Giving a reason, arrange these elements in the increasing order of atomic numbers in the period.
Solution :
(a) (i) All the elements of a group have similar chemical properties because they have same no. of valence electrons in their outermost shell.
(ii) All the elements of a period have different chemical properties because they have different no. of valence electrons in their atoms.
(b) Order of atomic numbers of elements: X < Z < Y. Because as the atomic number increases in a period from left to right, the size of atoms goes on decreasing
Question 26:
(a) How does the electropositive character of elements change on going down in a group of the periodic table ?
State how the valency of elements varies (i)in a group, and (ii)in a period, of the periodic table.
Solution :
(a) On going down in a group of the periodic table, the electropositive character of elements increases.
(b) (i) In a group, all the elements have the same valency.
(ii) In a period, on moving from left to right, the valency of elements first increases from 1 to 4 and then decreases to zero.
Question 27:
(a) What is the fundamental difference in the electronic configurations between the group 1 and group 2 elements ?
(b) On the basis of electronic configuration, how will you identify :
(i) chemically similar elements ?
(ii) the first element of a period ?
Solution :
(a) The fundamental difference between the electronic configuration of group 1 and group 2 elements is that group 1 elements have 1 valence electron in their atoms whereas group 2 elements have 2 valence electrons in their atoms.
(b) ( i ) All the chemically similar elements will have same valence electrons.
(ii) The 1st element in a period is determined by the no. of valence electrons in its atoms. The 1st element of every period has 1 valence electron.
Question 28:
(a) What is the usual number of valence electrons and valency of group 18 elements of the periodic table ?
(b) What happens to the number of valence electrons in the atoms of elements as we go down in a group of the periodic table ?
Solution :
(a) Usual number of valence elec trons is 8; Valency is 0 (zero).
(b) The number of valence electrons remains the same .
Question 29:
(a) What is the main characteristic of the last elements in the periods of the periodic table ? What is the general name of such elements ?
(b) What is the number of elements in : (a) 1st period, and (b) 3rd period, of the modern periodic table ?
Solution :
(a) The main characteristic of last elements in a period is that they all have 8 valence electrons in their atoms except helium. Such elements are called noble elements.
(b) (i) 2
(ii) 8.
Question 30:
(a) How does the atomic size vary on going down from top to bottom in a group of the periodic table ? Why does it vary this way ?
(b) Lithium, sodium and potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements ? Explain your answer.
Solution :
(a) On going down in a group of the periodic table, the atomic size increases. When we move from top to bottom in a group, a new shell of electrons is added to the atoms at every step due to which the size of atom increases.
(b) The similarity in the atoms of lithium, sodium and potassium is that all of them have 1 valence electron each.
Question 31:
(a) How does the tendency to lose electrons change as we go down in group 1 of the periodic table ? Why does it change this way ?
(b) How does the tendency to gain electrons change as we go down in group 17 of the periodic table ? Why does it change this way ?
Solution :
(a) The tendency of an atom to lose electrons increases on moving down in a group of the periodic table. As we go down in group 1, one more electron shell is added at every stage and the size of the atom increases. The valence electrons become more and more away from the nucleus and hold of the nucleus on valence electrons decreases. Due to this, the atoms can lose valence electrons more easily to form positive ions and hence electropositive character increases.
(b) The tendency of an atom to gain electrons decreases on going down in a group of the periodic table. When we move from top to bottom in group 17, a new shell of electrons is added to the atoms at every step, due to which the size of atom increases. The nucleus goes more deep inside the atom due to which the attraction of nucleus for the incoming electron decreases due to which the atom cannot form negative ions easily and hence the electronegative character decreases.
Question 32:
(a) Why does the size of the atoms progressively become smaller when we move from sodium (Na) to chlorine (Cl) in the third period of the periodic table ?
(b) Helium and neon are unreactive gases. What, if anything, do their atoms have in common ?
Solution :
(a) As we move from Na to Cl in the 3rd period, the size of the atoms of the elements decreases. Na atom is the biggest whereas Cl atom is the smallest in size. As we move from left to right in a period, the atomic no. of elements increases i.e. the number of protons and electrons in the atoms increases. Due to large positive charge on nucleus, the electrons are pulled in more close to the nucleus and thus the size of the atom decreases from Na to Cl.
(b) Helium and neon atoms have completely filled outermost electron shells (containing the maximum number of electrons which can be accommodated in them) .
Question 33:
(a) In the modern Periodic Table, why does cobalt with higher atomic mass of 58.93 appear before nickel having lower atomic mass of 58.71 ?
(b) Why could no fixed position be given to hydrogen in Mendeleev’s periodic table ?
Solution :
(a) Modern periodic table arranges the elements according to increasing atomic numbers. So, the atomic number of cobalt (27) comes first whereas the atomic number of nickel (28) comes later.
(b) In Mendeleev’s periodic table, hydrogen has been placed in group I since like alkali metals, hydrogen also combines with halogens, oxygen and sulphur to form compounds having similar formulae. This means that hydrogen resembles alkali metals in some of the properties.
Hydrogen also resembles halogens in some of the properties. So, hydrogen could also be placed in group VII of halogen elements.
Thus, Mendeleev’s periodic law could not assign a correct position to hydrogen in the periodic table.
Question 34:
(a) What are the periods and groups in a periodic table ? Give two characteristics of each.
(b) In terms of electronic configurations, explain the variation in the size of the atoms of the elements belonging to the same period and same group.
(c) Given alongside is a part of the periodic table. As we move vertically downward from Li to Fr :
(i) What happens to the size of atoms ?
What happens to their metallic character ?
(d) Name two properties of elements whose magnitudes change when going from top to bottom in a group of the periodic table. In what manner do they change ?
(e) Rewrite the following statement after correction, if necessary :
Groups have elements with consecutive atomic numbers.

Solution :
(a) The horizontal rows of elements in a periodic table are called periods.
Characteristics:
(i) The elements in a period have consecutive atomic numbers.
(ii) The no. of elements in period is fixed by the maximum no. of electrons which can be accommodated in various shells.
The vertical columns in a periodic table are called groups.
Characteristics:
(i) The elements in a group do not have consecutive atomic numbers.
(ii) All the elements in a group have similar electronic configurations and show similar properties.
(b) The size of atom decreases on moving from left to right in a period. As we move from left to right in a period, the atomic number of elements increases which means that the no. of protons and electrons in the atom increases. The electronic configuration of the atoms increases in the same shell. Due to large positive charge on the nucleus, the electrons are pulled in more close to the nucleus and the size of the atom decreases.
On going down in a group of the periodic table, the atomic size increases. The no. of electron shells in the atoms gradually increases and the electronic configuration also increases due to which the atomic size increases.
(c)(i) The atomic size increases gradually from lithium to francium.
(ii) The metallic character increases from lithium to francium.
(d) On going down in a group of the periodic table, the atomic size and metallic character increases. When we move down from top to bottom in group 1 of alkali metals, the size of atoms increases gradually from lithium to francium.
In group 1 of alkali metals, lithium is the least metallic element whereas francium is the most metallic element.
(e) Periods have elements with consecutive atomic numbers .
Lakhmir Singh Chemistry Class 10 Solutions Page No:305
Question 35:
(a) Explain why, the first period of the modern periodic table has only two elements whereas second period has eight elements
(b) Why do elements in the same group show similar properties but the elements in different groups show different properties ?
(c) For each of the following triads, name the element with the characteristics specified below :

(d) State one reason for keeping fluorine and chlorine in the same group of the periodic table.
(e) What are the merits of the modern periodic table of elements ?
Solution :
(a) The 1st period has two elements because the 1st electron shell of an atom c a n take a maximum of two electrons only. The 2nd period of the periodic table has 8 electrons because the maximum no. of electrons which can be put in the 2nd shell of an atom is 8.
(b) The elements in the same group show similar properties because they have similar electronic configuration (having the same number of valence electrons) whereas the elements of different groups have different electronic configurations (different number of valence electrons) due to which they show different properties.
(c) (i) F; Br
(ii) Li; Li
(d) Fluorine and chlorine have been placed in the same group because both of them have 7 valence electrons.
(e) Merits of modern periodic table:
(i) The modern periodic table is based on the atomic numbers of elements which is the most fundamental property of elements.
(ii) It helps us to understand why elements in a group show similar properties but elements in different groups show different properties.
(iii) It explains the reasons for the periodicity in properties of elements.
(iv) It tells us why the properties of elements are repeated after 2, 8, 18 and 32 elements.
Question 36:
(a) What is a group in the periodic table ? In which part of a group would you separately expect the elements to have (i) the greatest metallic character (ii) the largest atomic size ?
(b) In what respects do the properties of group 1 elements differ from those of group 17 elements ? Explain with examples by taking one element from each group.
(c) From the standpoint of atomic structure, what determines which element will be the first and which the last in a period of the periodic table ?
(d) Explain why, the properties of elements are repeated after 2, 8,18 and 32 elements in the periodic table.
(e) What are the advantages of the periodic table ?
Solution :
(a) The vertical columns in a periodic table are called groups.
(i) The greatest metallic character is found in the elements in the lowest part of the group.
(ii) The largest atomic size is found in the lowest part of the group.
(b) Group 1 elements have 1 valence electron and are ionic in chemical reactions. Their chemical reactivity increases down the group. They are electropositive in nature and it increases down the group.
Whereas, the elements of group 17 have 7 valence electrons. They all are non-metals. Their chemical reactivity decreases down the group. They are electronegative in nature and it decreases down the group.
(c) The no. of valence electrons in the atoms of elements decides which element will be the 1st element in a period and which will be the last in a period.
(d) The properties of elements are repeated after 2, 8, 18 and 32 elements in the periodic table because the electronic configurations of the elements are repeated in this manner.
(e) Advantages of the periodic table:
(i) It has made the study of chemistry systematic and easy.
(ii) It is easier to remember the properties of an element if its position in the periodic table is known.
(iii) The type of compounds formed by an element can be predicted by knowing its position in the periodic table.
(iv) It is used as a teaching aid in chemistry in schools and colleges.
Lakhmir Singh Chemistry Class 10 Solutions Page No:306
Question 57:
The atomic numbers of the three elements X, Y and Z are 2, 6 and 10 respectively.
Which two elements belong to the same group ?
Which two elements belong to the same period ?
Give reasons for your choice.
Solution :
(i) X and Z.
X and Z have zero valency hence they belong to same group: noble gases.
(ii) Y and Z.
Y: 2,4 and Z: 2,8 so, both of them belong to second period with two shells filled.
Question 58:
An atom has the electron structure of 2, 7.
(a) What is the atomic number of this atom ?
(b) To which of the following would it be chemically similar ?
7N, 15P, 17CI, isAr
(c) Why would you expect it to be similar ?
Solution :
(a) 9.
(b) 17 Cl.
(c) Both have the same number of valence electrons (7 electrons each) in their atoms.
Question 59:
Consider the following elements :
20Ca, gO, isAr, 16S, 4Be, 2He Which of the above elements would you expect to be :
(i) very stable ? (ii) in group 2 of the periodic table ?
(iii) in group 16 of the periodic table ?
Solution :
(i) 18 Ar and 2 He (Noble gases) .
(ii) 20 Ca and 4 Be (no. of valence electrons in each = 2).
(iii) 8 O and 16 S (no. of valence electrons in each = 6).
Question 60:
In each of the following pairs, choose the atom having the bigger size :
(a) Mg (At. No.12) or Cl (At. No. 17)
(b) Na (At. No. 11) or K (At. No. 19)
Solution :
(a) Mg since atomic size decreases from left to right in a period.
(b) K since atomic size increases on going down a group .
Question 61:
The atomic numbers of three elements A, B and C are given below :

(i) Which element belongs to group 18 ? (ii) Which element belongs to group 15 ?
(iii) Which element belongs to group 13 ? (iv) To which period/periods do these elements belong ?
Solution :
(i) C (2, 8).
(ii) B (2, 5).
(iii) A (2, 3).
(iv) 2nd period (2 shells are filled).
Question 62:
An element X belongs to 3rd period and group 2 of the periodic table. State :
(a) number of valence electrons (b) valency (c) metal or non-metal (d) name of the element
Solution :
(a) 2.
(b) 2 .
(c) Metal .
(d) Magnesium .
Lakhmir Singh Chemistry Class 10 Solutions Page No:307
Question 63:
The following diagram shows a part of the periodic table in which the elements are arranged according to their atomic numbers. (The letters given here are not the chemical symbols of the elements):

(i) Which element has a bigger atom, a or ƒ ?
(ii)Which element has a higher valency, k or o?
(iii) Which element is more metallic, i or k?
(iv) Which element is more non-metallic, d or g?
(v) Select a letter which represents a metal of valency 2.
(vi) Select a letter which represents a non-metal of valency 2.
Solution :
(i) a (size decreases from left to right in a period) .
(ii) k (valency of k = 3; valency of o = 1).
(iii) i (metallic character decreases from left to right in a period).
(iv) g (non-metallic character increases from left to right in a period) .
(v) b (or j) .
(vi) f (or n) .
Question 64:
An element X is in group 2 of the periodic table :
(a) What will be the formula of its chloride ?
(b) What will be the formula of its oxide ?
Solution :
Valency of X = 2
(a) XCl 2
(b) XO
Question 65:
An element Y is in second period and group 16 of the periodic table :
(i) Is it a metal or non-metal ?
(ii) What is the number of valence electrons in its atom ?
(iii) What is its valency ?
(iv) What is the name of the element ?
(v) What will be the formula of the compound formed by Y with sodium ?
Solution :
(i) Non-metal .
(ii) 6 .
(iii) 2 .
(iv) Oxygen .
(v) Na 2 Y .
Question 66:
(a) An element X has mass number 40 and contains 21 neutrons in its atom. To which group of the periodic table does it belong ?
(b) The element X forms a compound X2Y. Suggest an element that Y might be and give reasons for your choice.
Solution :
(a) Group 1 (2, 8, 8, 1).
(b) Oxygen (X is monovalent so Y has to be divalent to form the compound X2Y)
Question 67:
An element X combines with oxygen to form an oxide XO. This oxide is electrically conducting.
(a) How many electrons would be there in the outermost shell of the element X ?
(b) To which group of the periodic table does the element X belong ?
(c) Write the formula of the compound formed when X reacts with chlorine.
Solution :
(a) 2.
(b) Group 2 .
(c) XCl 2 .
Question 68:
An element A has an atomic number of 6. Another element B has 17 electrons in its one neutral atom.
(a) n which groups of the periodic table would you expect to find these elements ?
(b) What type of bond is formed between A and B ?
(c) Suggest a formula of the compound formed between A and B.
Solution :
(a) A in group 14; B in group 17.
(b) Covalent bond.
(c) AB 4 .
Question 69:
The elements A, B, C and D belong to groups 1, 2,14 and 17 respectively of the periodic table. Which of the following pairs of elements would produce a covalent bond ?
(i) A and D (ii)C and D (iii) Aand B (iv)B and C (v) Aand C
Solution :
C and D .
Question 70:
An element X from group 2 reacts with element Y from group 16 of the periodic table.
(a) What is the formula of the compound formed ?
(b) What is the nature of bond in the compound formed ?
Solution :
(a) XY.
(b) Ionic bond .
Question 71:
A metal X is in the first group of the periodic table. What will be the formula of its oxide ?
Solution :
V alency of group 1 metals is 1 so it will react with oxygen (valency = 2) to form X2O.
Question 72:
An element A from group 14 of the periodic table combines with an element B from group 16.
(i) What type of chemical bond is formed ?
(ii) Give the formula of the compound formed.
Solution :
(i) Covalent bond is formed between two non-metals (A and B).
(ii) AB 2 .
Question 73:
An element X from group 2 of the periodic table reacts with an element Y from group 17 to form a compound.
(a) What is the nature of the compound formed ?
(b) State whether the compound formed will conduct electricity or not.
(c) Give the formula of the compound formed.
(d) What is the valency of element X ?
(e) How many electrons are there in the outermost shell of an atom of element Y ?
Solution :
(a) Ionic compound.
(b) Yes .
(c) XY 2 .
(d) 2 .
(e) 7 .
Lakhmir Singh Chemistry Class 10 Solutions Page No:308
Question 74:
The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters a, b, c, d and e (which are not their chemical symbols):

(i) Select the letter which represents an alkali metal.
(ii) Select the letter which represents a noble gas.
(iii) Select the letter which represents a halogen.
(iv) What type of bond is formed between a and e?
(v) What type of bond is formed between d and e ?
Solution :
(i) d.
(ii)c.
(iii) e.
(iv) Covalent bond .
(v) Ionic bond .
Question 75:
The elements A, B and C belong to groups 1, 14 and 17 respectively of the periodic table.
(a) Which two elements will form a covalent compound ?
(b) Which two elements will form an ionic compound ?
Solution :
(a) B and C.
(b) A and C .
Question 76:
Find the neutral atom in the periodic table which has the same number of electrons as K+ and Cl . What is this number ?
Solution :
Argon atom, 18 electrons .
Question 77:
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electron shells but different
number of electrons in their outermost shells. It was found that elements A and G combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during
cooking. Oxides of elements A and B are basic in nature while those of elements E and F are acidic. The
oxide of element D is, however, almost neutral. Based on the above information, answer the following questions :
(a) To which group or period of the periodic table do these elements belong ?
(b) What would be the nature of compound formed by a combination of elements B and F ?
(c) Which two of these elements could definitely be metals ?
(d) Which one of the eight elements is most likely to be found in gaseous state at room temperature ?
(e) If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
Solution :
(a) 3rd period.
(b) Ionic compound .
(c) A and B.
(d) H.
(e) CG3 .
Question 78:
Write the names and symbols of two very reactive metals belonging to group 1 of the periodic table. Explain by drawing electronic structure, how either one of the two metals reacts with a halogen. With which name is the bond formed between these elements known and what is the class of the compound so formed known ? State any four physical properties of such compounds.
Solution :
Sodium (Na) and Potassium (K);
Sodium (Na) is a metal. So, sodium readily reacts with a halogen like chlorine (Cl) to form an ionic chloride called sodium chloride. This is illustrated below:
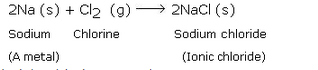
Ionic bond; Ionic compounds .
Physical properties of ionic compounds:
(i) Ionic comp ounds are usually hard, brittle.
(ii) They conduct electricity when molten or dissolved.
(iii) They have high melting and boiling points.
(iv) Most are soluble in polar solvents such as water.
Question 79:
The non-metal A is an important constituent of our food and most of the fuels around us. A forms two oxides B and C. The oxide B is poisonous whereas oxide C causes global warming.
(a) Identify A, B and C.
(b) To which group of periodic table does A belong ?
(c) Name another element which is placed in the same group as A.
Solution :
(a) A is carbon (C); B is carbon monoxide (CO) ; C is carbon dioxide (CO 2 ).
(b) 14 th group .
(c) Silicon (Si) .
Question 80:
A non-metal X which is the largest constituent of air combines with hydrogen when heated in the presence of iron as catalyst to form a gas Y. When gas Y is treated with sulphuric acid, it forms a compound Z which is used as a chemical fertiliser.
(a) What are X, Y and Z ?
(b) To which group of periodic table does X belong ?
(c) Name the period of periodic table in which X is placed.
(d) Which element is placed just before X in the period ?
(e) Which element is placed just after X in the period ?
Solution :
(a) X is nitrogen gas, N 2 ;
Y is ammonia gas, NH 3
and Z is ammonium sulphate, (NH 4)2SO4.
(b) 15 th group .
(c) 2 nd period.
(d) Carbon, C .
(e) Oxygen, O.
You can watch video lessons on YouTube: https://www.youtube.com/user/cbsepapers/videos
More Resources for CBSE Class 10:
Lakhmir Singh Class 10 Physics