S Chand Biology Class 10 Solutions Model Test Paper 3
Question 1.
Why is the red color selected for danger signal lights?
Answer:
The wavelength of red light is the longest in visible light, so the red color is scattered the least by air molecules of the atmosphere and therefore it can reach to a longer distance. That’s why the red color is used for danger signal lights.
Question 2.
Balance the following chemical equation.
MnO2 + HCl → MnCl2 + Cl2 + H2O
Answer:
Balanced chemical equation.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Question 3.
What is the common name of CaSO4. \(\frac{1}{2}\)H2O ? Also name the compound from which it is prepared.
Answer:
The common name of CaSO4. \(\frac{1}{2}\)H2O is plaster of paris. Plaster of paris is obtained by heating gypsum (CaSO4.2H2O) at 373 K.
Question 4.
What is the resultant resistance of the three resistors of 5 Ω, 4 Ω and 10 Ω connected as shown in the figure? What is this combination of resistors known as?
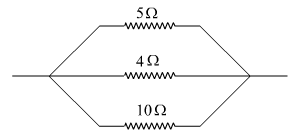
Answer:
This given combination of resistors is known as parallel combination.
In a parallel combination, the reciprocal of the resultant resistance is equal to the sum of reciprocals of the individual resistances present in the combination. For the three resistors connected in parallel,
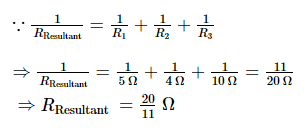
Question 5.
Define variation in relation to a species. Why is variation beneficial to the species?
Answer:
The occurrence of differences among the individuals of the same species is known as variation.
Variation helps in the survival of species by bringing about changes in the basic body design, thereby providing the organisms with a better adaptability against predators.
Question 6.
(a) Show on a diagram the transfer of electrons between the atoms in the formation of MgO.
(b) Name the solvent in which ionic compounds are generally soluble.
(c) Why are aqueous solutions of ionic compounds able to conduct electricity?
Answer:
(a) Transfer of electrons between atoms in the formation of magnesium oxide.
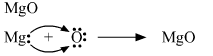
(b) Water is the best solvent in which ionic compounds are generally soluble.
(c) The aqueous solution of ionic compounds can conduct electricity because of the presence of free ions. These ions are responsible for the conduction of electricity in aqueous solutions.
Question 7.
Explain why, the blue colour of copper sulphate solution fades when an iron nail is kept dipped in it. Write chemical equation of the reaction involved. What does this reaction tell us about the relative reactivities of copper and iron?
Answer:
When an iron nail is put in the blue colour copper sulphate solution, then the iron displaces copper from copper sulphate and forms green coloured iron sulphate solution, that’s why the blue colour of the solution fades. The reaction involved is as follows.
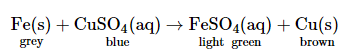
As iron displaces copper from its sulphate solution, so, we can conclude that iron is more reactive than copper.
Question 8.
Why are decomposition reactions called the opposite of combination reactions? Explain by giving one example (with equation) of each type of reaction.
Answer:
Decompositon reaction are those in which a compound breaks down to form two or more substances. These reaction require a source of energy to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
Decomposition reaction. \(2 \mathrm{H}_{2} \mathrm{O} \stackrel{\text { electrolysis }}{\longrightarrow} 2 \mathrm{H}_{2}+\mathrm{O}_{2}\)
Combination reaction. 2H2 + O2 → 2H2O + energy
Question 9.
The atom of an element has the electron structure 2, 7.
(a) What is the atomic number of the element?
(b) To which of the following would it be chemically similar?
7N, 15P, 17Cl, 18Ar
(c) Why would you expect it to be similar?
Answer:
(a) The atomic number of the element is 9.
(b) Out of given options, 17Cl has similar properties with this element.
Electronic configuration of Cl is 2, 8, 7. So, valence shell contains 7 electrons.
(c) The modern periodic table assumes that elements with the same number of valence electrons have similar properties.
Question 10.
The position of hand shown in the figure corresponds to one of the Fleming’s rules.

(a) Which Fleming’s rule is illustrated by this hand?
(b) In this figure of hand, what is indicated.
(i) by the direction of forefinger?
(ii) by the direction of center finger?
(iii) by the direction of thumb?
Answer:
(a) Fleming’s left-hand rule is illustrated by the hand.
(b) (i) The magnetic field is indicated by the direction of fore-finger.
(ii) Electric current is indicated by the direction of the center finger.
(iii) By the direction of thumb, the motion of the conductor is indicated in the given figure.
Question 11.
Two resistors, with resistances 5 Ω and 10 Ω respectively are to be connected to a battery of emf 6 V so as to obtain. (i) minimum current flowing (ii) maximum current flowing.
(a) How will you connect the resistances in each case?
(b) Calculate the strength of the total current in the circuit in two cases.
Answer:
(a) In a parallel combination of resistances, the net resistance is less as compared to a series combination of resistances. As the current flow is indirectly proportional to the resistance of the circuit, so to obtain maximum current flowing in the circuit the given resistances should be connected in parallel combination and to obtain the minimum current flowing the given resistances should be connected in series combination.
(b) Case (i) For minimum current flow, the resistance of the circuit should be maximum. i.e. resistances should be connected in Series.
Net resistances of given resistances when connected in series = 5 Ω + 10 Ω = 15 Ω
The voltage of the battery = 6 V
Hence, current = \(\frac{6 \mathrm{V}}{15 \Omega}\) = 0.4 A
Case (ii) For maximum current flow, the resistance of the circuit should be minimum. i.e. resistances should be connected in parallel.
Net resistances of given resistances when connected in parallel = \(\frac{5 \Omega \times 10 \Omega}{5 \Omega+10 \Omega}=\frac{10}{3} \Omega\)
The voltage of the battery = 6 V
Hence, current = \(\frac{6 \mathrm{V}}{\frac{10}{3} \Omega}\) = 1.8 A
Question 12.
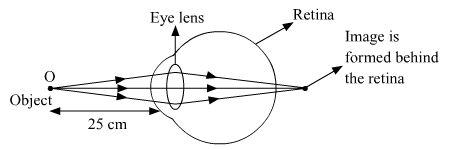
What is hypermetropia? State the two causes of hypermetropia. With the help of ray diagrams, show.
(a) the eye-defect hypermetropia
(b) correction of hypermetropia by using a lens
Answer:
Hypermetropia or long-sightedness is a common eye defect, in which a person is not able to see nearby objects whereas the farther objects appear clear to the person.
Two causes of hypermetropia.
(i) Eyeball being too short.
(ii) The increased focal length of the eye lens.
(a) Hypermetropic eye.
(b) Correction made with a convex lens for the hypermetropic eye.
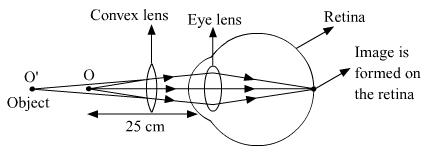
Question 13.
Distinguish between biodegradable and non-biodegradable substances. List two effects of each of them on our environment.
Answer:
|
Biodegradable Substance |
Non-biodegradable Substance |
| It decomposes naturally in the environment by the action of microorganisms. |
It does not decompose naturally. |
| It is environment friendly. | It is harmful to the environment and causes pollution. |
| It is made up of natural ingredients. | It is made up of synthetic materials. |
| It can be converted into manure or recycled. | It can be either reused or recycled. |
| Examples: Waste paper, wood crumbles, etc. | Examples: Plastic bags, cans, disposable bottles, etc. |
Effects of biodegradable substances on environment –
- Decomposition of biodegradable wastes is accompanied by foul smell which spreads in the environment and affects the people in nearby areas.
- Heaps of biodegradable wastes act as breeding grounds for houseflies etc, which act as vectors of various diseases.
Effects of non-biodegradable substances on environment –
- Excessive use of non-biodegradable pesticides and fertilizers affect the fertility of soil.
- Certain non-biodegradable wastes enter the food chains, get biomagnified and affect the various biotic components of environment.
Question 14.
Why are bacteria and fungi called decomposers? List any two advantages of decomposers to the environment.
Answer:
Bacteria and fungi are called decomposers as they obtain nutrients by breaking down the remains of dead plants and animals.
Role of decomposers
- They help in the breakdown of organic matter or biomass of dead plants and animals into simple inorganic raw materials such as CO2, H2O and nutrients.
- They help in the natural replenishment of soil.
Question 15.
(a) Name two different ways in which glucose is oxidized to provide energy in various organisms.
(b) Write any two differences between the two ways of oxidation of glucose in organisms.
Answer:
(a) Glucose can undergo either aerobic or anaerobic respiration to provide energy.
(b)
|
Anaerobic respiration |
Aerobic Respiration |
|
|
(i) |
It involves the partial breakdown of glucose. | It involves the complete breakdown of glucose into CO2 and H2O. |
|
(ii) |
A net gain of only 2 molecules of ATP occurs. | A net gain of 36 molecules of ATP occurs. |
Question 16.
(a) Which two criteria did Mendeleev use to classify the elements in his periodic table?
(b) State Mendeleev’s periodic law.
(c) Why could no fixed position be given to hydrogen in Mendeleev’s periodic table?
(d) How and why does the atomic size vary as you go.
(i) from left to right along a period?
(ii) down a group?
Answer:
(a) Two criteria used by Mendeleev in creating his periodic table.
(i)Mendeleev’s periodic table was based on the observation that the properties of elements are the function of their atomic masses. This means that if elements are arranged in the increasing order of their atomic masses, then their properties get repeated after regular intervals.
(ii) Relative atomic mass and similarity of chemical properties.
(b) Mendeleev’s periodic law states that the physical and chemical properties of elements are the periodic function of their atomic weights.
(c) hydrogen has atomic mass 1 so definitely, it must be in the first period but the group was confusing. He found that hydrogen forms oxides like alkali earth metals with formula H2O as Na2O. He also found that hydrogen is similar to halogens in forming diatomic molecules H2 like Cl2, Br2. So, he could not assign a fixed position to hydrogen.
(d)
(i) On moving left to right in a period, atomic size decreases because of the number of electron increases due to which attractive forces towards nucleus increases and atomic size decreases.
(ii) On moving down the group, atomic size increases due to the increase in valence shells.
Question 17.
Write the names and symbols of the two most reactive metals belonging to the Group I of the periodic table. Explain by drawing electronic structure how either one of the two metals reacts with a halogen. With which name is the bond formed between these elements known and what is the class of the compound so formed known? State any four physical properties of such compounds.
Answer:
The two most reactive elements of group 1 are cesium (Cs) and rubidium(Rb). Since valence shell of cesium contains only 1 electron, so it can lose it and form positive Cs+ ion. The electron lost by cesium can be accepted by halogen atom like chlorine and form negative chloride ions. The positive and negative ions attract each other to form electrostatic bond also called ionic bond. This type of compound formed is called an ionic compound.
Electronic configuration of cesium chloride.
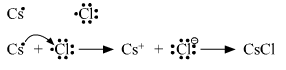
Properties of the ionic compound are.
- High melting and boiling point.
- Good electrical conductivity in the molten state and aqueous solution.
- Physical state is usually crystalline solid.
- Soluble in water.
Question 18.
(a) What is a solenoid? Draw a sketch of the pattern of field lines of the magnetic field through and around a current carrying solenoid.
(b) Consider a circular loop of wire lying in the plane of the table. Let the current pass through the loop clockwise. Apply the right hand rule to find out the direction of the magnetic field inside and outside the loop.
Answer:
(a) A solenoid is a long coil that contains a large number of close turns of insulated copper wire. The magnetic field pattern produced by a current-carrying solenoid is similar to the magnetic field produced by a bar magnet.
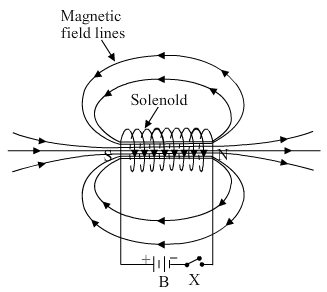
(b)
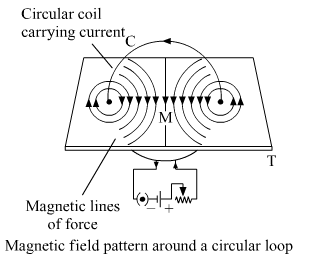
We can apply the right-hand thumb rule to determine the direction of magnetic field lines around a current caring circular wire.
Here, the current is flowing into the clockwise direction, hence the direction of magnetic field lines inside the loop is going inward the table and the direction of the magnetic field outside the loop is coming out of the table. As shown in the above figure.
Question 19.
Derive the expression for the heat produced to a current ‘I’ flowing for a time interval ‘t’ through a resistor ‘R’ having a potential difference ‘V’ across its ends. With which name is the relation known? How much heat will an instrument of 12 W produce in one minute if it is connected to a battery of 12 V?
Answer:
Heat energy = power consumed × time
∴ H = P × t
Let ‘I’ is the current flowing for a time interval ‘t’ through a resistor ‘R’ having a potential difference ‘V’ across its ends.
Power = I2R or \(\frac{V^{2}}{R}\)
∴ H = I2Rt or \(\frac{V^{2}}{R} t\)
This relation is known as Joule’s law of heating.
Power of the instrument = 12 W
Time for which the instrument is used = 1 minute = 60 Seconds
Heat produced = P × t = 12 W × 60 s = 720 J
Question 20.
(a) Draw a diagram of human alimentary canal and label on it.
Oesophagus, Gall bladder, Liver and Pancreas
(b) Explain the statement, ‘Bile does not contain any enzyme but it is essential for digestion.’
Answer:
(a)
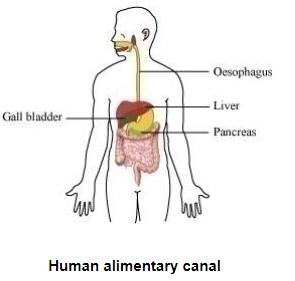
(b) Bile does not contain any enzyme, but it plays an important role in digestion as it performs the following functions.
- The bile salts break down large fat globules into smaller globules so that the pancreatic enzymes can easily act on them. This is referred to as emulsification of fats.
- The food entering the small intestine is acidic which has to be made alkaline so that the pancreatic enzymes can act on it. This function is also performed by bile juice.
Question 21.
(a) Draw a diagram of excretory system in human beings and label on it.
Aorta, vena cava, urinary bladder, urethra.
(b) List two vital functions of the kidney.
Answer:
(a)
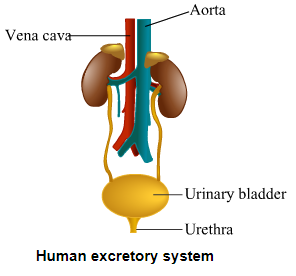
[Note. Aorta and vena cava are components of the circulatory system.]
(b) The two vital functions of kidney are.
- filters the wastes out of the blood and forms urine
- maintains the water balance of the body
Question 22.
Draw the following diagram in your answer-book and show the formation of the image of the object AB with the help of suitable rays.
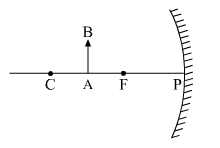
What type of reflecting surface is shown in the above diagram?
Answer:
Image formation is as follows.
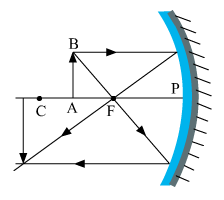
The above-shown reflecting surface is a converging surface of a concave mirror.
Question 23.
A student was given three resistors of 2 ohms, 3 ohms and 5 ohms by his teacher. The teacher asked him to connect these three resistors in such a way so as to obtain the minimum resistance.
(a) In what way should the student connect the resistors?
(b) Draw a diagram of the combination of resistors.
Answer:
In a parallel combination, the resultant resistance is always less than or equal to the smallest resistance in the combination. So, a parallel combination of resistances gives the minimum resistance.
(a) Hence, in order to get minimum resistance, the student needs to arrange them in a parallel combination.
(b) A parallel combination of given resistors is shown below.
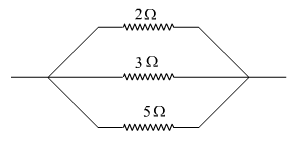
Question 24.
When a student adds some dilute hydrochloric acid to baking soda taken in a test-tube, a gas is evolved.
(a) Name the gas evolved.
(b) How can this gas be tested?
Answer:
When a student add dilute hydrochloric acid to baking soda(NaHCO3), the aqueous solution of sodium chloride, carbon dioxide gas, and water are obtained.
HCl(aq) + NaHCO3(aq) → NaCl(aq) + CO2(g) + H3O(l)
(a) So, the gas evolved is carbon dioxide.
(b) When we pass this gas from lime water, it turns the lime water milky and its presence is confirmed.
Question 25.
A student was given two test-tubes A and B each containing a colourless solution. When a few drops of universal indicator were added to the two test-tubes, one by one, then the colour of solution in test-tube A changed to blue whereas the colour of solution in test-tube B changed to orange.
(a) Which test-tube has a solution of pH higher than 7?
(b) Which test-tube has a solution of pH lower than 7?
(c) Which test-tube has an acidic solution?
(d) Which test-tube has an alkaline solution?
Answer:
(a) The universal indicator changes its color towards violet in basic or alkaline medium. This, the pH of the solution in test tube A is greater than 7, that’s why the universal indicator shows blue colour.
(b) The universal indicator changes its color towards red in acidic medium. So, the pH of the solution in test tube B is less than 7, that’s why the universal indicator is giving an orange colour.
(c) The pH of the solution in the test tube B is less than 7, so the test tube “B” has an acidic solution.
(d) The pH of the solution in test tube A is greater than 7, so test tube “A” has an alkaline solution.
Question 26.
A student conducted an experiment to show that carbon dioxide is released during respiration. State two precautions that the student must take for obtaining correct observations.
Answer:
Two precautions which should be taken during performing the experiment to show that carbon dioxide is produced during respiration are
- The conical flask should be air tight so that the CO2 produced during the experiment does not escape and a partial vacuum can be created in the flask.
- Germinating seeds should be used in the experiment because germinating seeds produce CO2 which has to be tested in this experiment.
Question 27.
A star-shaped figure was cut in the black paper strip used for covering a part of the leaf of a destarched plant used for demonstrating that light is necessary for photosynthesis. At the end of the experiment (after the removal of chlorophyll), the leaf was tested for starch with iodine solution.
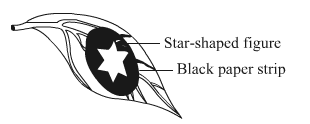
(a) What colour, if any, will be produced on the part of leaf under the star-shaped figure?
(b) Give reason for your answer.
Answer:
(a) The part of leaf under the star region will show blue black colour upon testing with iodine.
(b) The appearance of blue colour in that part indicates the presence of starch.