Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 9 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 9 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
The correct IUPAC name of the coordination compound K3[Fe(CN)5NO] is [1]
(a) potassium pentacyanonitrosylferrate (II)
(b) potassium pentacyanonitroferrate (II)
(c) potassium nitritopentacyanoferrate (IV)
(d) potassium nitritepentacynanoiron (II)
Answer:
(a) potassium pentacyanonitrosylferrate (II)
IUPAC name of K3[Fe(CN)5NO] is potassium pentacyanonitrosylferrate (II).
Question 2.
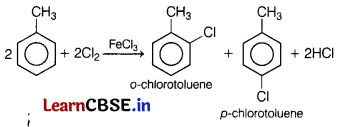
The reaction of toluene with chlorine in presence of FeCl3 gives predominantly [1]
(a) a mixture of o-and p-chlorotoluene
(b) benzyl chloride
(c) m-chlorotoluene
(d) benzoyl chloride
Answer:
(a) a mixture of o-and p-chlorotoluene
Toluene gives o-chlorotoluene, p-chlorotoluene and hydrogen chloride as products when reacts with chlorine in the presence of FeCl3.
Question 3.
Which of the following statements concerning methylamine is correct? [1]
(a) Methylamine is stronger base than NH3.
(b) Methylamine is less basic than NH3.
(c) Methylamine is slightly acidic.
(d) Methylamine forms salts with alkali.
Answer:
(a) Methylamine is stronger base than NH3.
Methylamine is more basic than NH3. Due to +I-effect of methyl group, the electron density of nitrogen atom increases due to which basic nature increases.
![]()
Question 4.
Match the properties with the elements of 3d series [1]
| (i) Element with the highest melting point | (p) Ni |
| (ii) Element that forms the most stable oxide | (q) Cr |
| (iii) Element with the highest ionization energy | (r) Fe |
| (s) Co |
(a) (i)-(q), (ii)-(p), (iii)-(r)
(b) (i)-(p), (ii)-(q), (iii)-(r)
(c) (i)-(r), (ii)-(p), (iii)-(s)
(d) (i)-(q), (ii)-(s), (iii)-(r)
Answer:
(a) (i)-(q), (ii)-(p), (iii)-(r)
The correct match is (i) – (q), (ii) – (p), (iii) – (r).
Question 5.
In 30 minutes, a first-order reaction is 50% complete. Calculate the amount of time it took to complete 87.5 percent of the reaction. [1]
(a) 30 minutes
(b) 60 minutes
(c) 90 minutes
(d) 120 minutes
Answer:
(c) 90 minutes
In 30 minutes reaction is 50% complete.
∴ t1/2 = 30 minutes
For first order reaction, t1/2 = \(\frac{0.6932}{k}\)
k = \(\frac{0.6932}{t_{1 / 2}}\) = \(\frac{0.6932}{30}\) = 0.02310 min-1
Now, time for 87.5% completion of reaction
k = \(\frac{2.303}{t}\)log\(\frac{a}{a-x}\)
0.02310 = \(\frac{2.303}{t}\)log\(\frac{100}{12.5}\)
t = 90 minutes
Question 6.
Which of the following is the right temperature coefficient (η) expression? [1]
(a) η = Rate constant at T + 10°C/Rate constant at T°C
(b) η =Rate constant at T + 20°C/Rate constant at T°C
(c) η =Rate constant at T = 30°C/Rate constant at T°C
(d) η = Rate constant at T + 40°C/Rate constant at T°C
Answer:
(a) η = Rate constant at T + 10°C/Rate constant at T°C
The effect of temperature on a reaction space is commonly stated in terms of temperature which is defined by equation.
(η) \(=\frac{T+10^{\circ} \mathrm{C}(308 \mathrm{~K}) \text { rate constant }}{T^{\circ} \mathrm{C} \text { rate constant }(298 \mathrm{~K})}\)
Question 7.
Which graph correctly correlates £ceu as a function of concentrations for the cell (for different values of M and M’)?
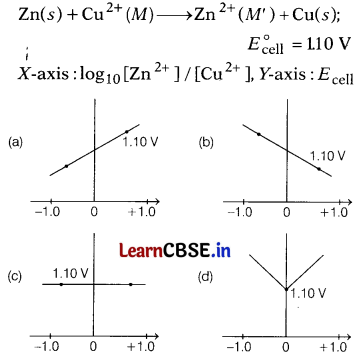
[1]
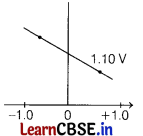
Answer:
(b)
According to Nernst equation,
Ecell = E°cell – \(\frac{0.059}{2}\)log\(\frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}\)
or y = c + (-m)x
The slope is negative.
Hence, graph (B) correctly correlates Ecell as a function of concentrations for the cell.
Question 8.
Which of the following graphs represents a first-order reaction? [1]
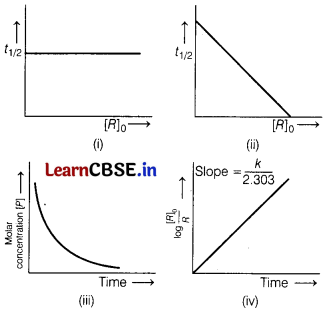
Choose the correct option,
(a) (i, ii)
(b) (ii, iii)
(c) (iii, iv)
(d) (i, iv)
Answer:
(d) (i, iv)
For first order reaction half-life (t1/2) is independent of initial concentration of reactant (R0). So, correct graph of t1/2 vs R0 is
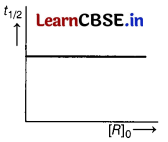
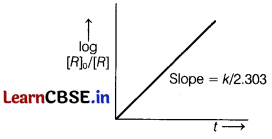
Also, for first order reaction, k = \(\frac{2.303}{t}\)log\(\frac{\left[R_0\right]}{[R]}\)
or log\(\frac{\left[R_0\right]}{[R]}\) = \(\frac{k t}{2.303}\)
∴ Correct graph of log\(\frac{\left[R_0\right]}{[R]}\)v/st
Question 9.
There is a large difference in the boiling points of butanal and butan-1 -ol due to [1]
(a) intermolecular hydrogen bonding in butan-1 -ol
(b) intermolecular hydrogen bonding in butanal
(c) higher molecular mass of butan-1-ol
(d) resonance shown by butanal
Answer:
(a) intermolecular hydrogen bonding in butan-1 -ol
Butanol has polar O—Hbond due to which it shows intermolecular H-bonding which is not possible in case of butanal due to absence of polar bond. Hence, butan-1-ol has higher boiling point than butanal.
![]()
Question 10.
Secondary amines can be prepared by [1]
(a) reduction of nitro compounds
(b) oxidation of N-substituted amides
(c) reduction of isonitriles
(d) reduction of nitriles
Answer:
(c) reduction of isonitriles
Isonitriles on reduction with LiAIH4 or catalytic hydrogenation (H2/ Ni) produces secondary amines.
![]()
Question 11.
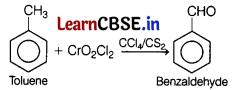
The oxidation of toluene to benzaldehyde by chromyl chloride is called [1]
(a) Etard reaction
(b) Riemer-Tiemann reaction
(c) Wurtz reaction
(d) Cannizzaro reaction
Answer:
(a) Etard reaction
The oxidation of toluene to benzaldehyde by chromyl chloride (CrO2Cl2) in presence of CCl4 or CS2 is known as Etard reaction.
Question 12.
The oxidation state of Cr in a [Cr(NH3)2F4]– complex is [1]
(a) 2
(b) 3
(c) 4
(d) 6
Answer:
(b) 3
Let oxidation state of Cr in [Cr(NH3)2F4]– be x.
So, x + 2(0) + 4(-1) = -1
x = -1 + 4; x = 3
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is followed by a corresponding Reason (R). Use the following keys to choice the appropriate answer.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 13.
Assertion (A) Aromatic primary amines can be prepared by Gabriel phthalimide synthesis.
Reason (R) Aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide. [1]
Answer:
(d) (A) is false, but (R) is true.
Aromatic primary amines cannot be prepared by Gabriel phthalimide synthesis because aryl halides don’t undergo nucleophillic substitution with anion formed by phthalimide. Hence, (A) is false, (R) is true.
Question 14.
Assertion (A) Hydrolysis of sucrose brings about a change in sign of rotation from dextro to laevo.
Reason (R) Hydrolysis always changes the optical rotation of a compound. [1]
Answer:
(c) (A) is true, but (R) is false.
Hydrolysis of sucrose brings about a change in sign from dextro to laevo. Sucrose is dextrorotatory but on hydrolysis, it gives equimolar amounts of glucose which is dextrorotatory and fructose is laevorotatory.
Since, the laevorotation of fructose (- 92.4°) is much more than dextrorotation of glucose (+ 52.5°) therefore, resulting solution becomes laevorotatory. Optical rotation doesn’t depend on hydrolysis. Hence, (A) is true but, (R) is false.
Question 15.
Assertion (A) The aqueous solution of FeCl3 is basic in nature.
Reason (R) FeCl3 hydrolyses in water. [1]
Answer:
(d) (A) is false, but (R) is true.
FeCl3 is acidic in nature due to hydrolysis.
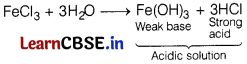
Hence, (A) is false but (R) is true.
Question 16.
Assertion (A) Reimer-Tiemann reaction of phenol with CCl4 in NaOH at 340 K gives salicyclic acid as the major product.
Reason (R) The reaction occurs through intermediate formation of trichlorocarbene. [1]
Answer:
(c) (A) is true, but (R) is false.
Yes, Reimer-Tiemann reaction of phenol with CCl4 in NaOH at 340 K gives salicylic acid as major product. Nucleophillic attack of phenolate ion through the oxygen to carbon atom occur on dichlorocarbene to form an intermediate, i.e. dichloromethyl substituted phenol which on hydrolysis gives salicylic acid. Hence, (A) is true, but (R) is false.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
Account for the following.
(a) Carbon atoms in glucose are linked in a straight chain.
(b) Amphoteric behaviour of amino acids. [2]
Answer:
(a) On prolonged heating with HI, it forms n-hexane, which shows that all the six carbon atoms are linked in a straight chain.
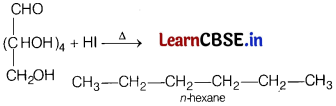
(b) In a single molecule, amino acids have both an acidic . (carboxyl group) and a basic (amino group). They neutralise each other in aqueous solutions. A proton is released by the carboxyl group, whereas it is accepted by the amino group.
Amino acids react with both acids and bases in their Zwitter ionic form, displaying amphoteric behaviour.
Or
What happens when D-glucose is treated with following reagents.
(a) Methyl alcohol in the presence of dry HCl gas.
Answer:
Glucose reacts with methyl alcohol in the presence of dry HCI gas forming methyl glycoside which is an ether.
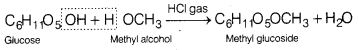
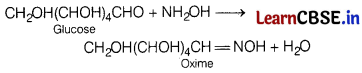
(b) Hydroxyl amine.
Answer:
Glucose reacts with hydroxylamine to form an oxime.
![]()
Question 18.
Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers? [2]
Answer:
[Pt(NH3) (Br)(Cl) (py)]
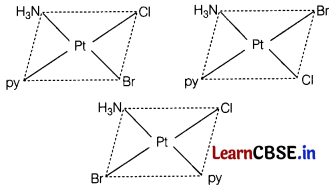
From the above isomers, none will exhibit optical isomers. Tetrahedral complexes rarely show optical isomerisation. They do so only in the presence of unsymmetrical chelating agents.
Question 19.
A reaction is of second order with respect to a reactant. How will the rate of reaction be affected if the concentration of this reactant is [2]
(a) doubled
(b) reduced to half?
Answer:
For second order reaction,
rate = k[A]2
let [A] = a, then rate = ka2
(a) If [A] = 2a, then rate = k(2a)2 = 4ka2
∴ Rate of reaction becomes 4 times
(b) If [A] = a/2, then rate = k (a/2) = ka2 / 4
∴ Rate of reaction will be 1/4th.
Question 20.
The rate constant for a reaction of zero order in A is 0.0030 mol L-1 s-1. How long will it take for the initial concentration of A to fall from 0.10 M to 0.075 M? [2]
Answer:
For zero order reaction, time (f) = \(\frac{1}{k}\)[A0 – A]
where, k = rate constant
= 0.0030 mol L-1s-1
= 0.10 M = 0.075 M
A0 = initial concentration of reactant
A = concentration of reactant at time f
t = \(\frac{1}{0.003}\)(0.10 – 0.075) = \(\frac{1}{0.003}\) × 0.025
t = 8.3 s
Question 21.
Give reason for the following. [2]
(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Answer:
Electron pairs of Cl atom are in conjugation with π electrons of the benzene ring so C—Cl bond in chlorobenzene acquires some double bond character, while C—Cl bond in cyclohexyl chloride is a pure single bond.
C— Cl bond in chlorobenzene is shorter than in cyclohexyl chloride. Since, dipole moment is a product of charge and distance, so chlorobenzene has lower dipole moment than cyclohexyl chloride.
(b) Alkyl halides, though polar, are immiscible with water.
Answer:
Alkyl halides are polar molecules, therefore, their molecules are held together by dipole-dipole attraction. Whereas, the molecules of H2O are held together by H-bonds.
Since, the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide- alkyl halide molecules and water- water molecules, therefore, alkyl halides are immiscible with water.
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
The air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% and 79% by volume at 298 K, respectively. The water is in equilibrium with air at a pressure of 10 atm. At 298 K, if the Henry’s law constant for oxygen and nitrogen are 3.30 × 107 mm and 6.51 × 107 mm respectively, calculate the composition of these gases in water. [3]
Answer:
Given that, total pressure of air in equilibrium with water = 1o atm
As air contains 20% oxygen and 79% nitrogen by volume.
∴ Partial pressure of oxygen
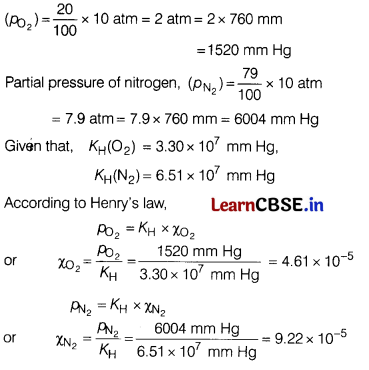
Question 23.
The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2mol-1. Calculate its degree of dissociation and dissociation constant.
Given, \(\lambda_{\left(\mathrm{H}^{+}\right)}^0\) = 349.6 S cm2 mol-1 and
\(\lambda_{\left(\mathrm{HCOO}^{-}\right)}^0\) = 54.6 S cm2 mol-1
[3]
Answer:
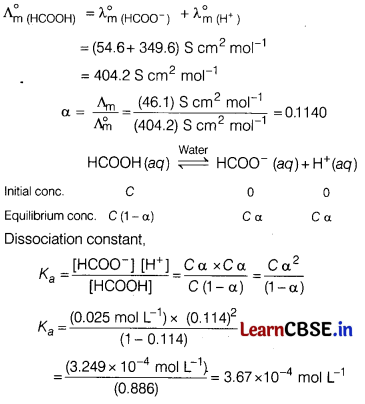
Question 24.
Write the equations involved in the following reactions.
(a) Phenol is treated with CHCl3 in the presence of aqueous NaOH at 340K.
(b) Ethyl bromide is treated with sodium ethoxide and phenol is treated with benzene diazonium salt. [3]
Answer:
(a) Treatment of phenol with CHCl3 in the presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.
This is a Reimer-liemanri reaction
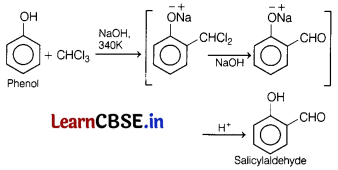
(b)
This reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism. This reaction is known as Williamson’s ether synthesis.
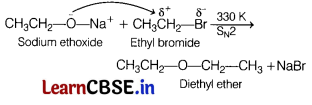
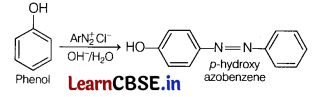
(c) Phenol is treated with benzene diazonium salt to give p-hydroxy azobenzene. This is an example of the coupling reaction.
Question 25.
Identify the major product formed when phenol reacts with carbon dioxide. Name the reagent which is used to carry out the reaction. Also, write the name of the reaction involved and explain why are alcohols more soluble in water than the hydrocarbons of comparable molecular masses? [3]
Answer:
Phenol reacts with CO2 in presence of reagent sodium hydroxide (NaOH) at 4-7 atm and 390- 410 K giving salicylic acid. This reaction is known as Kolbe’s reaction
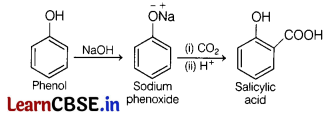
Alcohols can form H-bonds with water and break the H-bonds already existing between water molecules.
So, they are soluble in water.
On the other hand, hydrocarbons cannot form H-bonds with water and hence are insoluble in water.
Or
(a) Name the reactant that yields benzoquinone on oxidation with Na2Cr2O7 / H2SO4. Write the reactions involved.
Answer:
Phenol forms benzoquinone on oxidation with Na2Cr2O7 /H2SO4.
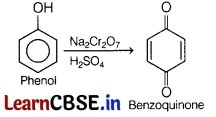
(b) Why are phenols more soluble in water than benzene of comparable molecular masses?
Answer:
Phenols are more soluble in water than benzene of comparable molecular masses due to the presence of the hydroxyl (—OH) group attached to the aromatic ring in phenols. The hydroxyl group in phenols provides a polar functional group that allows phenols to form hydrogen bonds with water molecules. This hydrogen bonding between phenols and water enhances the solubility of phenols in water compared to benzene, which cannot form such strong hydrogen bonding intractions.
Question 26.
For the complex [Fe(en)2Cl2]Cl,
(en = ethylene diamine), identify
(a) the oxidation number of iron.
(b) the hybridisation and the shape of the complex.
(c) the magnetic behaviour of the complex. [3]
Answer:
(a) [Fe(en)2Cl2]Cl
Let the oxidation state of Fe be
x + (2 × 0)+ 2(-1) + (-1) = 0
x + (- 3) = 0 or x = + 3
∴ Oxidation number of iron, x = + 3.
(b) The complex has two bidentate ligands and two monodentate ligands.
Therefore, the coordination number is 6 and hybridisation will be d2sp3 and shape will be octahedral.
(c) In the complex, 26Fe3+ = 3d54s04p0
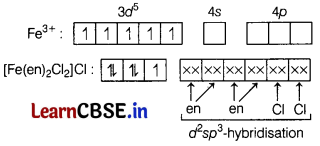
Due to the presence of one unpaired electrons in d-orbitals the complex is paramagnetic.
![]()
Question 27.
Give reasons for the following observations. [3]
(a) Aniline is a weaker base than cyclohexyl amine.
Answer:
In aniline, the lone pair of electrons on the N-atom is delocalised over the benzene ring. As a result, the electron density on the nitrogen decreases.
But in cyclohexylamine, the lone pair of electrons on N-atom is readily available due to the absence of π-electrons. Hence, aniline is weaker base than cyclohexylamine.
(b) It is difficult to prepare pure amines by ammonolysis of alkyl halides.
Answer:
Because the primary amine formed by ammonolysis itself acts as a nucleophile and produces further 2° and 3° alkyl amine.
(c) Electrophilic substitution in aromatic amines takes place more readily than benzene.
Answer:
Due to the strong activating effect of the NH2 group, aromatic amines undergo electrophilic substitution reactions more readily than benzene.
Question 28.
Give reasons for the following [3]
(a) The presence of —NO2 group at ortho position increases reactivity of haloarenes towards nucleophilic substitution reactions.
Answer:
—NO2 shows -I and -M effect when present at ortho and para position w.r.t. halogens.
It has tendency to attract the electrons towards itself, thus they decreases the electron density between
![]()
(X = halogens) bond. Therefore —NO2 at ortho and para positions w.r.t. halogens in haloarenes increases the reactivity.
(b) p-dichlorobenzene has higher melting point than the ortho or meta-isomer
Answer:
p-dichlorobenzene has higher melting point than that of ortho or meta-isomers due to its symmetrical structure, which makes it a more compact compound and increases the melting point.
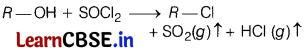
(c) Thionyl chloride method is preferred for preparing alkyl chloride from alcohols.
Answer:
Thionyl chloride (SOCl2) is preferred for the preparation of alkyl chloride from alcohol, because the by-products we get in this reaction are escapable gases, thus we get good yield of alkyl-chloride, i.e.
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties. Meena has performed an experiment in which she prepared a sugar solution and inferred the data given in table below.
| Lowering in vapour pressure | 0.061 mm of Hg |
| Vapour pressure of water 20°C | 17.5 mm of Hg |
Answer the following questions. [4]
(a) What will be the relative lowering of vapour pressure for the given solution?
Answer:
Vapour pressure of water (p°A) = 17.5 mm of Hg
Lowering of vapour pressure (p°A – pA) = 0.061
Relative lowering of vapour pressure
= \(\frac{p_A^{\circ}-p_A}{p_A^{\circ}}\)
= \(\frac{0.061}{17.5}\) = 0.00348
Or
Find the vapour pressure (mm of Hg) of solution.
Answer:
Vapour pressure of solution (pA) = Vapour pressure of solvent (p°A) – lowering of vapour pressure (p°A – pA)
pA = 17.5 – 0.061 = 17.439 mm of Hg
(b) What will be the mole fraction of sugar in the solution?
Answer:
Relative lowering of vapour pressure is given as
\(\frac{\rho_A^{\circ}-p_A}{p_A^{\circ}}\) = χB {XB = mole fraction of sugar (solute)}
\(\frac{0.061}{17.5}\) = χB ⇒ 0.00348 = χB
Hence, mole fraction of sugar in solution = 0.00348.
(c) What will be the vapour pressure (mm of Hg) of water at 293 K when 25g of glucose is dissolved in 450 g of water?
Answer:
Relative lowering of vapour pressure is given as
\(\frac{p_A^{\circ}-p_A}{p_A^{\circ}}\) = χB
or \(\frac{p_A^{\circ}-p_A}{p_A^{\circ}}\) = \(\frac{W_B}{M_B}\) × \(\frac{M_A}{W_A}\)
WB = weight of sugar
MB = molecular mass of sugar
MA = molecular mass of water
WA = weight of water
\(\frac{17.5-p_A}{17.5}\) = \(\frac{25}{450}\) × \(\frac{18}{180}\)
17.5 – pA = 17.5 × 5.56 × 10-3
17.5 – pA = 0.0973 ⇒ pA = 17.40 mm of Hg
Question 30.
Enzymes are Composed of one or several polypeptide chains. However, there are a number of cases in which non-protein constituents called co-factors are bound to the enzyme to make the enzyme catalytically active. In these instances, the protein portion of the enzymes is called the apoenzyme. Three kinds of cofactors may be identified: prosthetic groups, co-enzymes and metal ions. Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are tightly bound to the apoenzyme.
For example, in peroxidase and catalase, which catalyse the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a part of the active site of the enzyme. Co-enzymes are also organic compounds but their association with the apoenzyme is only transient, usually occurring during the course of catalysis.
Furthermore, co-enzymes serve as co-factors in a number of different enzyme catalysed reactions. The essential chemical components of many coenzymes are vitamins, e.g. coenzyme nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin. A number of enzymes require metal ions for their activity which form coordination bonds with side chains at the active site and at the same time form one or more coordination bonds with the substrate, e.g. Zinc is a cofactor for the proteolytic enzyme carboxypeptidase. Catalytic activity is lost when the co-factor is removed from the enzyme which testifies that they play a crucial role in the catalytic activity of the enzyme.
Answer the following questions. [4]
(a) Zinc is a co-factor for the proteolytic enzyme carboxypeptidase. What happens if co-factor is removed from the enzyme?
Answer:
Catalytic activity is lost when the co-factor is removed from the enzyme which testifies that they play a crucial role in the catalytic activity of the enzyme.
(b) Co-factor play a crucial role in the catalytic activity of the enzyme. Enlist the type of co-factor with examples,
Answer:
Three kinds of co-factors are there
Prosthetic groups – e.g.
Haem Co-enzymes – e.g. Niacin
Metal ions – e.g. Zinc
(c) Explain competitive inhibition and inhibitor.
Answer:
When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor. Due to its close structural similarity with the substrate, the inhibitor competes with the substrate for the substrate binding site of the enzyme. Consequently, the substrate cannot bind and as a result, the enzyme action declines. This phenomenon is called as competitive inhibition.
Or
Describe the functions of enzymes, and name two diseases which are caused due to the deficiency of enzymes. [4]
Answer:
Enzymes are highly specific in nature. The two of main factors are as follows
- They lower the requirement of activation energy.
- They speed up the rate of reaction.
e.g. Enzyme catalyses maltose to glucose.
Two diseases which are caused due to the .deficiency of enzymes are
- Phenylketonuria
- Albinism
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
Attempt any five of the following questions. [5]
(a) Why there is more variability in oxidation states of transition metals than that of the p-block elements?
Answer:
Variability in oxidation states in transition metals is due to the partially filled d-orbitals and comparable energy gaps between s, p and d-orbitals. It allows electron transfer between oxidation states consequently leading to maximum oxidation state possible.
While in p-block elements, octet rule is followed because of their poor shielding effect and being further from the nucleus. That is why in p-block, oxidation states varies by 2 unit while in transition metals, only, 1 unit changes are observed, e.g.
S(+2), S(+4), S(+6)Fe+2, Fe+3, Co+2, Co+3, etc.
(b) Why is Cu2+ (aq) is much more stable than Cu+(aq)?
Answer:
Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionisation enthalpy of copper is large but ∆hydH for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionisation enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows
2Cu+ → Cu2+ + Cu
(c) Why orange colour of \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) ion changes to yellow when treated with an alkali?
Answer:
The orange coloured potassium dichromate solution when treated with basic NaOH solution, is converted to chromate which gets a faint colour like yellow. The reaction is given as
k2Cr2O7 + 2NaOH → K2CrO4 + Na2CrO4 + H2O
This formation of chromate (\(\mathrm{CrO}_4^{2-}\)) ion converts the colour of the solution to yellow.
(d) Explain why chemistry of actinoids is complicated as compared to lanthanoids?
Answer:
- Lanthanoids show limited oxidation states, i.e. + 2, + 3, + 4 out of which + 3 is most common which is due to large energy gap between 4 f and 5d-subshells while actinoids show large number of oxidation states due to small energy gap between 5f, 6d and 7s subshells.
- Actinoid contraction is greater than lanthanoid contraction due to poor shielding of 5f electrons.
- Actinoids are radioactive in nature.
(e) Write two consequences of lanthanoid contraction.
Answer:
Consequences of lanthanoid contraction
(i) Similarity in properties Due to lanthanoid contraction, the size of elements which follow (Hf- Hg)are almost similar to the size of the elements, of previous row (Zr – Cd)and hence these are difficult to separate.
Due to small change in atomic radii, the chemical properties of lanthanoids are very similar due to which separation of lanthanoid becomes very difficult.
(ii) Basicity difference Due to lanthanoid contraction, the size decreases from La+3 to Lu+3. Thus, covalent character increases. Hence, basic character of hydroxides also decreases, that’s why La(OH)3 is most basic, while Lu(OH)3 is least basic.
(f) The lowest oxide of a transition metal is basic, the highest is amphoteric or acidic.
Answer:
In lower oxidation states, transition metals behave like metals and metal oxides are basic in nature.
Thus, in lower oxidation states, transition metal oxides are basic.
As the oxidation state increases, its metallic character decreases due to decrease in size, thus, it becomes less metallic or more non-metallic. Oxides of a non-metal may be acidic or neutral. Thus, in higher oxidation states, transition metal oxides are amphoteric or acidic.
(g) Cobalt (II) is stable in aqueous solution but in the presence of complexing agents, it is easily oxidised.
Answer:
In the presence of complexing agents, cobalt gets oxidised from +2 to +3 state because it provides energy to remove an electron from CO2+. Moreover, Co (III) is more stable than Co (II).
![]()
Question 32.
(a) An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid also produces (B). On dehydration (C) gives but-l-ene. Write the equations for the reactions involved.
(i) Identify A, B and C.
(ii) Write all the chemical reactions involved.
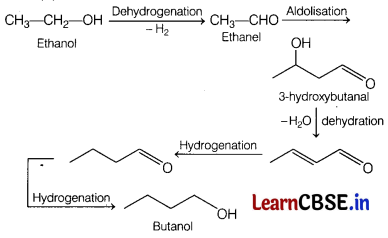
(iii) Write down the reaction to prepare C from ethanol. [5]
Answer:
(i) Since, the organic compound (A) with molecular formula (C8H16O2) was hydrolysed with dilute sulphuric acid and gives carboxylic acid (B) along with alcohol (C) therefore, it must be an ester. Further since oxidation of (C) with chromic acid produces the acid (6), therefore both the carboxylic acid (S) and the alcohol (C) must contain the same number of carbon atoms. So, (A) will be butyl butanoate, (B) will be butanoic acid and (C) will be butanol.
(ii)
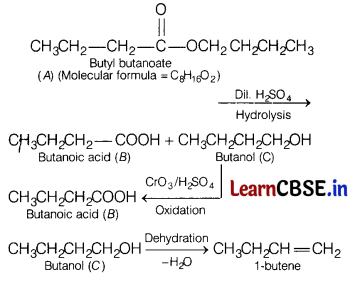
(iii)
(b) Account for the following :
(i) Aromatic carboxylic acid do not undergo Friedel-Crafts reaction.
(ii) pKa value of 4-nitrobenzoic acid is lower than that of benzoic acid.
Answer:
(i) The — COOH group attached to benzene ring is electron withdrawing in nature and thus deactivates ring. Secondly, the catalyst used in the reaction, i.e. AlCl3 is a Lewis acid which has the tendency to get bonded to carboxyl group. That’s why aromatic carboxylic acid does not undergo Friedel Crafts reaction.
(ii) Lower pKa value means stronger acid, pKa value of 4-nitrobenzoic acid is lower than benzoic acid which is due to -I and – R effect of — NO2 group.
Or
An aromatic compound ‘A’ (molecular formula C8H8O) gives a positive 2, 4-DNP test. It gives a yellow precipitate of compound ‘B’ on treatment with iodine and sodium hydroxide solution. Compound A’ does not give Tollen’s or Fehling test. On severe oxidation with potassium permanganate forms a carboxylic acid ‘C (Molecular formula C7H6O2), which is also formed alongwith the yellow compound in the above reaction. [5]
Write the chemical equation involved in the formation of A, B and C. Explain which one is more acidic among A or C. Also write one method of preparation of A from benzene.
Answer:
A will be acetophenone, B will be iodoform (yellow precipitate) and C will be benzoic acid.
Acetophenone will give 2,4-DNP test. It will react with 2,4-dinitrophenylhydrazine and form its derivative called the 2,4-dinitrophenylhydrazone.
Also, acetophenone gives a positive iodoform test by giving a yellow precipitate of iodoform with an alkaline solution of iodine.
In the presence of alkaline KMnO4, acetophenone is oxidised to benzoic acid.
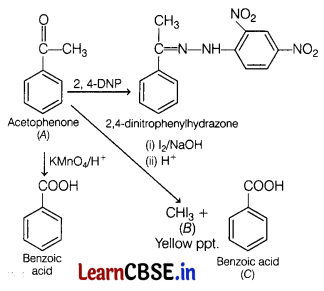
Benzoic acid (C) is more acidic than acetophenone (A) because of resonance stabilisation of their carboxylate ion formed in benzoic acid.
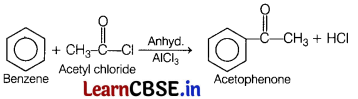
Acetophenone can be prepared by Friedel-Crafts acylation. Acetyl chloride reacts with benzene in the presence of anhydrous aluminium chloride to form acetophenone.
Question 33.
(a) Conductivity of 2.5 × 10-4 M methanoic acid is 5.25 × 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation.
Given: \(\Lambda_{\left(\mathrm{H}^{+}\right)}^{\circ}\) = 349.5 S cm2 mol-1 and
\(\Lambda_{\left(\mathrm{HCOO}^{-}\right)}^{\circ}\) = 50.5 S cm2mol-1. [5]
Answer:
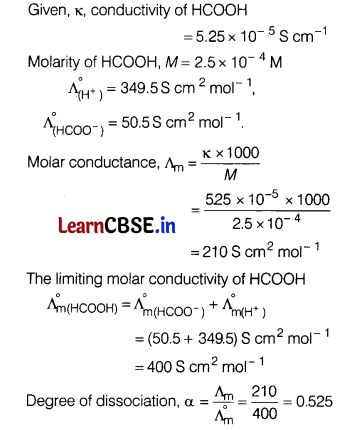
(b) Write the name of the cell which is generally used in transistors. Write the reactions taking place at the anode and the cathode of this cell.
Answer:
Leclanche cells (dry cell) is used in transistors.
At anode,
Zn(s) → Zn2+ + 2e–
At cathode.
2MnO2 + 2N\(\mathrm{H}_4^{+}\) + 2e– → Mn2O3 + 2NH3 + H2O
Or
(a) Define molar conductivity of a substance and describe how for weak and strong electrolytes, molar conductivity changes with concentration of solute. How is such change explained? [5]
Answer:
Molar conductivity of a solution at a given concentration is entire conductance of the volume ‘V’ of a solution containing one mole of electrolyte kept between two electrodes with area of cross section A and distance of unit length. It is represented by λm (lamda).
In case of strong electrolytes there is a small increase in conductance with dilution because a strong electrolyte is completely dissociated in solution and the number of ions remain constant.
Moreover, there will be greater interionic attractions at higher concentrations which retards the motion of
ions and conductance decreases.
In case of weak electrolytes there is an increase in conductance with decrease in concentration due to the increase in the number of ions in the solution.
(b) What is the dissociation constant of acetic acid if \(\Lambda_{\mathrm{m}}^{\circ}\) for acetic acid is 390 S cm2 mol-1 and the conductivity of 0.00 1 M acetic acid is 4 × 10-5 S/m? [4]
Answer:
Conductivity \((\mathbf{K})\) = 4 × 10-5 S cm-1
Concentration (C) = 0.001 M
Limiting molar conductivity \(\lambda_m^0\) = 390 s cm2 mol-1
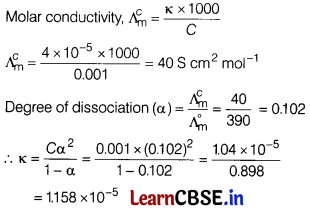
(c) What would be the voltage of a voltaic cell at 25°C with the following half cells:
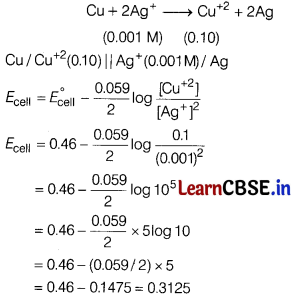
Ag+(0.001 M) | Ag and Cu2+(0.10 M) Cu . (E°cell = 0.46 V)
Answer:
The reaction takes place in cell as