Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 7 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 7 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
The general electronic configuration of transition element is transition element is [1]
(a) (n – 1)d1-10
(b) (n – 1)d10ns2
(c) (n – 1)d1-10ns1-2
(d) (n – 1)d5ns1
Answer:
(c) (n – 1)d1-10ns1-2
The general electronic configuration of transition elements is (n – 1)d1-10ns1-2
Question 2.
The oxidation state of Fe in [Fe(H2O)5NO]SO4 is [1]
(a) +1
(b) +2
(c) +4
(d) +3
Answer:
(a) +1
[Fe(H2O)5NO]SO4
Let the oxidation state of Fe be x.
∴ x + 5 × 0 + 1 – 2 = 0
+1 + x – 2 = 0
x = +1
![]()
Question 3.
Arrange the following in increasing order of their boiling points. [1]
A : Ethylamine
B : Diethylamine
C : N, N-dimethyl ethylamine
(a) C < B < A
(b) A < B < C
(c) B < C < A
(d) B < A < C
Answer:
(a) C < B < A
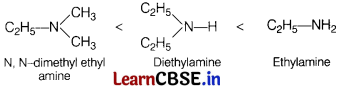
Increasing extent of hydrogen bonding increasing boiling point.
Question 4.
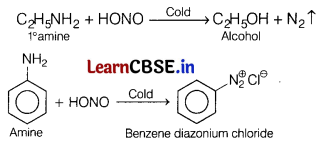
Which of the following is not correct? [1]
(a) Ethyl amine and aniline both have —NH2.
(b) Ethyl amine and aniline dissolve in HCl.
(c) Ethyl amine and aniline both react with CHCl3 and KOH to form unpleasant smelling compound.
(d) Ethyl amine and aniline both react with HNO2 in cold to give hydroxy compounds.
Answer:
(d) Ethyl amine and aniline both react with HNO2 in cold to give hydroxy compounds.
Nitrous acid reacts differently with ahphatic and aromatic amines ¡n cold.
Question 5.
The molar conductivities at infinite dilution of AgNO3 is 0.01334 mho m2 mol-1. Using the graph and given information, the molar conductivity of AgCl will be [1]
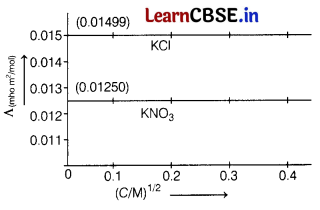
(a) 0.01583 mho m2 mol-1
(b) 0.01 mho m2 mol-1
(c) 0.01853 mho m2 mol-1
(d) 0.0125 mho m2 mol-1
Answer:
(a) 0.01583 mho m2 mol-1
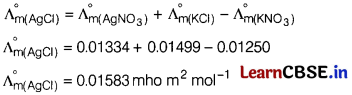
Question 6.
Match the following IUPAC names given in Column I with their common names given in Column II and choose the correct option from the codes given below. [1]
| Column I (IUPAC name) | Column II (Common name) |
| A. 4-methylphenol | 1. Catechol |
| B. Benzene-1, 4-diol | 2. Quinol |
| 3. Benzene-1, 2 diol | 3. o-cresol |
| 4. 2-methylphenol | 4. p-cresol |
Codes
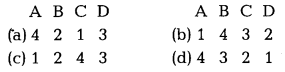
Answer:
(a)
![]()
The correct match is A – 4, B – 2, C – 1, D – 3.
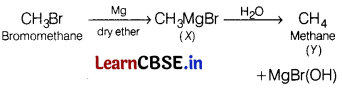
Question 7.
In the following reaction [1]
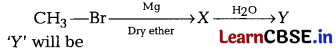
(a) CH4
(b) CH3MgBr
(c) CH3OH
(d) CH3 —CH3
Answer:
(a) CH4
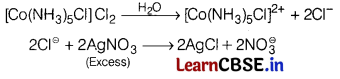
Question 8.
When one mole of C0Cl3 ∙ 5NH3 was treated with excess of silver nitrate solution, 2 moles of AgCl was precipitated. The formula of the compound is [1]
(a) [Co(NH3)5C]2]Cl
(b) [Co(NH3)5Cl]Cl2
(c) [Co(NH3)4Cl2](NH3)Cl
(d) [Co(NH3)Cl3]
Answer:
(b) [Co(NH3)5Cl]Cl2
2 mol AgCl will be obtained when 2 mol \(\mathrm{Cl}^{\ominus}\) ions are free in the solution.
Question 9.
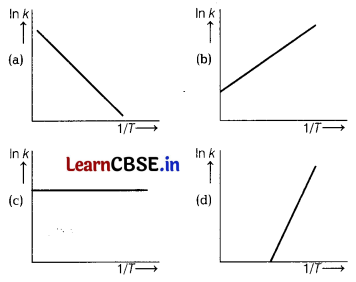
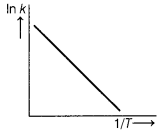
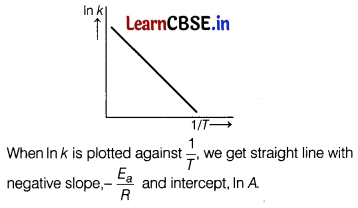
According to Arrhenius equation, rate constant k is equal to A\(e^{-E_a / R 7}\), which of the following options represents the graph of ln k vs \(\frac{1}{T}\) ? [1]
Answer:
(a)
![]()
Question 10.
Effervescence takes place when sodium carbonate solution is added to [1]
(a) formaldehyde
(b) acetaldehyde
(c) acetic acid
(d) phenol
Answer:
(c) acetic acid
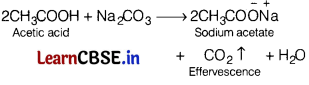
Question 11.
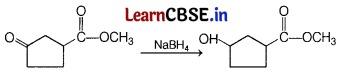
Consider the following reaction. [1]
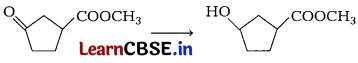
Which of the following reagent should be used to carry out the above conversion?
(a) LiAlH4
(b) NaBH4
(c) Zn|Hg|HCl
(d) KMnO4
Answer:
(b) NaBH4
Carbonyl group can be reduced by NaBH4. It is a weak reducing agent and does not affect ester group.
Question 12.
Lucas reagent produces cloudiness immediately with [1]
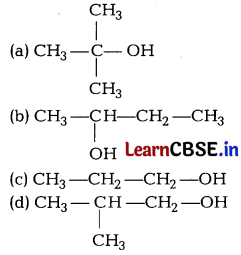
Answer:
(a)
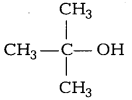
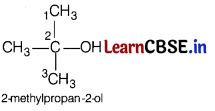
3° alcohols are most reactive towards Lucas reagent and thus produces cloudiness immediately.
Direction (Q. Nos. 13-16) In the following questions an Assertion (A) is followed by a corresponding Reason (R) use the following keys to choose the appropriate answer.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 13.
Assertion (A) K2Cr2O7 is orange-red crystalline compound.
Reason (R) Charge transfer develops colour in K2Cr2O7. [1]
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
K2Cr2O7 is orange-red crystalline solid. The colour in K2Cr2O7 develops because of the charge transfer.
Question 14.
Assertion (A) The C—O—C bond angle in ethers is slightly less than tetrahedral angle.
Reason (R) Due to the repulsive interaction between the two alkyl groups in ethers. [1]
Answer:
(d) (A) is false, but (R) is true.
The C—O—C bond angle in ethers is more than tetrahedral angle due to repulsive interactions between two alkyl groups.
Question 15.
Assertion (A) Sucrose is a non-reducing sugar.
Reason (R) It has glycosidic linkage. [1]
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true, but (R) is the correct explanation of (A).
Sucrose is a non-reducing sugar because its aldehydic group of glucose (C1) is bonded to ketonic group of fructose (C2).
Question 16.
Assertion (A) Hinsberg method is used in the separation of 1°, 2° and 3° amines.
Reason (R) Benzene sulphonyl chloride is used as Hinsberg reagent. [1]
Answer:
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
Both (A) and (R) are true, but (R) is not the correct explanation of (A).
Hinsberg test is used to distinguish 1°, 2° and 3° amines. The reagent (Hinsberg reagent) used for this purpose is benzene sulphonyl chloride.

Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
(a) A first order reaction takes 30 minutes for 50% completion. Calculate the time required for 90% completion of this reaction. [2]
Answer:
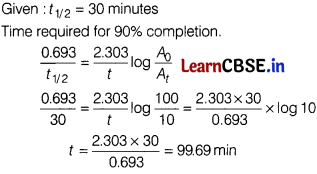
(b) Why does the rate of a reaction not remain constant throughout the reaction process?
Answer:
As r ∝ [Concentration]order
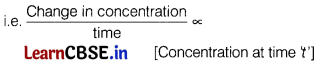
Hence, as time increases rate decreases.
Question 18.
Arrange the following complexes in increasing order of conductivity of their solutions. [2]
[Co(NH3)3Cl3], [Co(NH3)4Cl2]Cl,
[Co(NH3)6]Cl3, [Co(NH3)5Cl] Cl2
Answer:
Greater is the ionic concentration in the solution, more will be the conductivity. Therefore,
[Co(NH3)3Cl3] < [Co(NH3)4Cl2] Cl < [Co(NH3)5Cl]Cl2 < [Co(NH3)6]Cl3
Question 19.
Explain [2]
(a) rate of reaction increases with increase in temperature.
Answer:
According to Arrhenius hypothesis, the increase in the rate of reaction with increase in temperature is mainly due to increase in the kinetic energy of the reacting molecules which results in increased number of effective collisions.
(b) The role of catalyst on the rate of the reaction.
Answer:
A catalyst is a chemical substance which influences the rate of the reaction. A positive catalyst increases the rate whereas a negative catalyst decreases the rate of the reaction.
Question 20.
(a) Out of o-and p-dibromobenzene, which one has higher melting point and why? [2]
Answer:
p-dibromobenzene is symmetrical hence, its molecules are tightly packed in solid state. Therefore, it involves stronger intermolecular forces as compared to o-dibromobenzene. This is the reason why, the melting point of p-dibromobenzene is greater than o-dibromobenzene.
(b) Identify the products A and B formed in the following reaction.
CH3—CH2— CH = CH—CH3 + HCl → A + B
Answer:
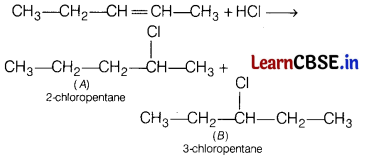
Question 21.
Account for the following. [2]
(a) The linkage connecting monosaccharide units in polysaccharides.
Answer:
Polysaccharides are polymers of monosaccharides. Monosaccharide units are connected each other by glycosidic linkage (etherial bond).
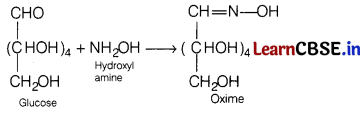
(b) The presence of an aldehydic group in glucose molecule.
Answer:
Glucose forms oxime on treatment with hydroxyl amine. This reaction confirms the presence of aldehydic group in glucose,
Or
What happens when D-fructose is treated with the following reagents. [2]
(a) Phenyl hydrazine
Answer:
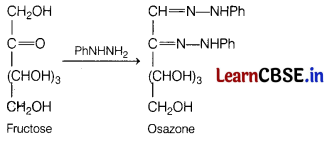
The reaction between fructose and PhNHNH2 gives osazone as a product.
(b) HNO3
Answer:
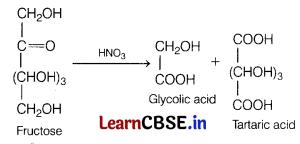
On reacting nitric acid with fructose, glycolic acid and tartaric acid are formed.
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
Identify the product formed when benzene diazonium chloride is treated with HBF4 followed by heating. Also explain why ethyl iodide undergoes SN2 reaction faster than ethyl bromide? [3]
Answer:
(a)
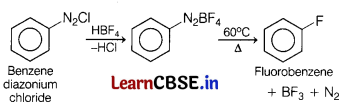
(b) C—I bond is weaker than C—Br bond hence, C2H5—I reacts faster than C2H5—Br in SN2 reaction.
Or
(a) Name the product formed when chloroform is treated with aniline in presence of alc. KOH. [3]
Answer:
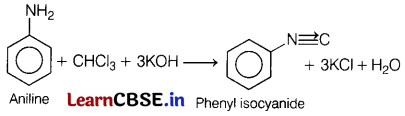
(b) Halogens are deactivating groups, though they are ortho-para directing. Why?
Answer:
Halogens are o, p-directing because of their + M-effect. But they are deactivating because of the strong -I-effect.
![]()
Question 23.
Write the equation for the following reactions. [3]
(a) Butan-2-ol is treated with alkaline KMnO4 solution.
Answer:
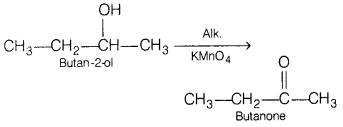
(b) Ethanol is treated with alkaline KMnO4 solution.
Answer:
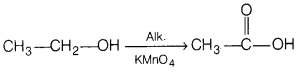
(c) Phenol is treated with acidified K2Cr2O7.
Answer:
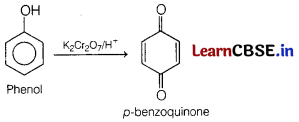
Question 24.
Give reasons for the following observations. [3]
(a) Role of HNO3 in the nitrating mixture used for nitration of benzene.
Answer:
In nitrating mixture, HNO3 acts as Bronsted base in presence of H2SO4 during the nitration of benzene.
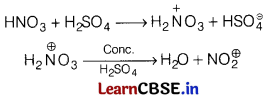
(b) Benzene diazonium chloride is not stored and is used immediately after its preparation?
Answer:
Benzene diazonium chloride is highly reactive hence, it cannot be stored. It is used immediately after its preparation.
(c) MeNH2 is stronger base than MeOH.
Answer:
MeNH2 is stronger base than MeOH because nitrogen is less electronegative than oxygen hence, electron pair of —NH2 group can be easily protonated.
Question 25.
Using VBT, explain the following in relation to the complex, [NiCl4]2-. [3]
(a) Type of hybridisation
(b) Magnetic moment value
(c) Type of complex-inner or outer orbital complex
Answer:
[NiCl4]2-
Oxidation state of Ni = + 2
Ni2+ = [Ar]3d84s0
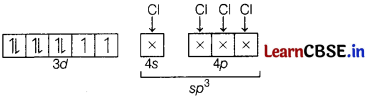
(a) Hybridisation-sp3
(b) No. of unpaired electrons =2
Magnetic moment, µ = \(\sqrt{2(2+1)}\)
= \(\sqrt{6}\) = 2.8 BM
(c) The complex is outer orbital complex.
Question 26.
Answer the following questions. [3]
(a) Explain why are aquatic species more comfortable in cold water in comparison to warm water?
Answer:
Aquatic species are more comfortable in cold water due to the presence of more oxygen. Solubility of oxygen in water increases with decrease in temperature as solubility of a gas in given liquid decreases with increase in temperature.
(b) Henry’s law constant for the molality of methane in benzene at 298 K is 427 × 105 mm Hg.
Calculate the solubility of methane in benzene at 298 K under 760 mm Hg.
Answer:
KH = 4.27 × 105 mm Hg (at 298 K)
p = 760 mm
Applying Henrys law,
P = KHχ.
where, x = mole traction or solubility of methane.
χ = \(\frac{p}{K_H}\) = \(\frac{760 \mathrm{~mm}}{427 \times 10^5 \mathrm{~mm}}\)
χ = 1.78 × 10-3
Question 27.
What type of battery is lead storage battery? Write the anode and cathode reactions, and the overall cell reaction occurring in the operation of a lead storage battery. [3]
Answer:
Lead storage battery is a secondary cell, Thus, it can be recharged by passing direct current through it.
Therefore, it can be reused. It is used in automobiles. In a lead storage cell, the anode is made of spongy lead and the cathode is a grid of lead packed with lead dioxide. The electrolyte used is H2SO4 (38% by mass).
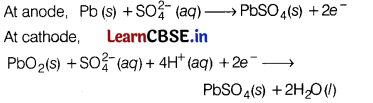
The overall cell reaction
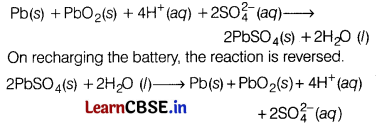
Question 28.
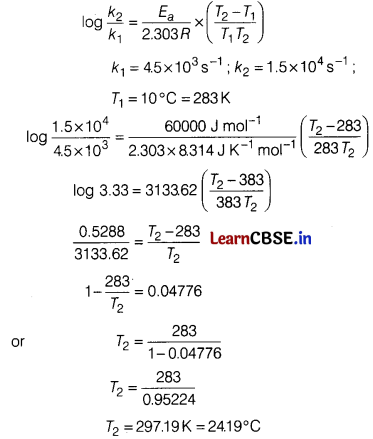
The decomposition of A into product has values of k as 4.5 × 103 s-1 at 10°C and energy of activation 60 kJ mol-1. At what temperature would k be 1.5 × 104 s-1? [3]
Answer:
According to Arrhenius equation,
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
Another class of biomolecules which are essential for living system, are proteins. Proteins are the most abundant biomolecules of the living system. The chief sources of protein are milk, cheese pulse, fish, meat, peanuts, etc.
They are found in every part of the body and form a fundamental basis of structure and functions of life. These are also required for growth and maintenance of body. Chemically proteins are the polymers in which the monomeric units are the a-amino acids.
Amino acids contain amino and carboxyl functional groups. Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified group, the amino acids can be classified as α, β, γ, or δ.
Answers the following questions. [4]
(a) Amino acids show amphoteric behaviour. Why?
(b) What type of linkage is responsible for the formation of protein?
(c) What is the difference between a-helix and (3-pleated sheets and give an example for each of fibrous protein and globular protein?
Answer:
(a) Amino acid contains both amino and carboxyl groups thus they react with both acid and bases.
Hence, amino acid are amphoteric in nature.
(b) Peptide linkage.
(c) In α-helix, a polypeptide chain forms all possible hydrogen bonds by twisting into a right handed helix.
On the other hand, in β-pleated sheets, peptide chains are stretched out to nearby maximum.
Extension and laid side by side intermolecular hydrogen bonds.
Fibrous protein Keratin and myosin.
Globular protein Insulin and albumin.
Or
What are the common type of secondary structure of protein? [4]
Answer:
The conformation which the polypeptide chain assume due to hydrogen bonding between
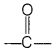
bond and —NH-group of the peptide bond is called secondary structure of protein.
These exist in two types of different structure
- α-helix and
- β-pleated structure.
Question 30.
The colligative properties of electrolytes require a slightly different approach than the one used for the colligative properties of non-electrolytes. The electrolytes dissociate into ions in a solution. It is the number of solute particles that determines the colligative properties of a solution. The electrolyte solutions, therefore, show abnormal colligative properties. To account for this effect we define a quantity called the van’t Hoff factor, given by
Actual number of particles is solution

i = 1 (for non-electrolytes);
i > 1 (for electrolytes, undergoing dissociation)
i < 1 (for solutes, undergoing association),
van’t Hoff factor depends on degree of dissociation or degree of association as the case may be.
Answer the following questions. [4]
(a) Give example of a solution in which solute molecules undergo association in solution.
Answer:
Berizoic acid undergoes dimerisation when dissolved in benzene.
3A → A3; i = 1/3
Or
A substance trimerises when dissolved in water, what will be its van’t Hoff factor? [4]
(b) Which of the following will have highest van’t Hoff factor? (Assume 100% dissociation)
C6H12O6, NaCl, CaCl2, Ca3(PO4)2
(c) 0.1 M K4[Fe(CN)6] is 60% dissociated. Calculated its van’t Hoff factor.
Answer:
(b) The van’t Hoff factor follows the order
Ca3(PO4)2 > CaCl2 > Nacl > C6H12O6
(c) K4[Fe(CN)6] \(\rightleftharpoons\) 4K+ + [Fe(CN)6]4-
α = \(\frac{i-1}{n-1}\)
0.60 = \(\frac{i-1}{5-1}\) ∴ i = 34
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
Answer the following. [5]
(a) For the reaction,
2AgCl(s) + H2(g) (1 atm) → 2Ag(s) + 2H+ (0.1 M) + 2Cl–(0.1 M),
∆G° =— 43600 J at 25°C.
Calculate the emf of the cell.
[log 10-n = -n]
Answer:
Gibbs energy of reaction is
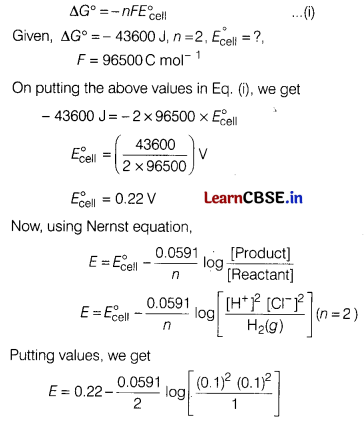

(b) What mass of Ag (At. mass 108) could be plated on a spoon from electrolysis of AgNO3 solution by one ampere current for 10 minutes?
Answer:
W = \(\frac{\mathrm{ItE}}{96500}\) = \(\frac{1 \times 10 \times 60 \times 108}{96500}\) = 0.6715 g
Or
(a) Define term molar conductance. [5]
Answer:
Molar conductance It is defined as the conductance of an electrolyte solution containing one mole of electrolyte.
\(\Lambda_m\) = \(\kappa\) × V = \(\frac{\kappa \times 1000}{M}\)
Units → ohm-1 m2 mol-1
(b) How Nernst equation can be applied in the calculation of equilibrium constant of any cell reaction?
Answer:
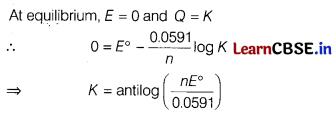
(c) Calculate the emf of the cell.
Cr|Cr3+(0.1 M) || Fe2+ (0.01 M)|Fe
Given: \(E_{\mathrm{Cr}^{3+} / \mathrm{Cr}}^{\circ}\) = -0.75 V,
\(E_{\mathrm{Fe}^{2+} / \mathrm{Fe}}^{\circ}\) = -045V
Answer:
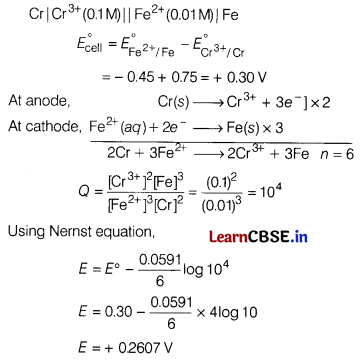
![]()
Question 32.
An alkene ‘A’ (molecular formula C5H10) on ozonolysis gives a mixture of two compounds B and C. Compound B gives positive Fehling test and also forms iodoform on treatment with I2 and NaOH.
Compound C does not give Fehling test but forms iodoform.
Identify A, B, C. Also write the reaction of ozonolysis of A and give iodoform test for B and C.
Answer:
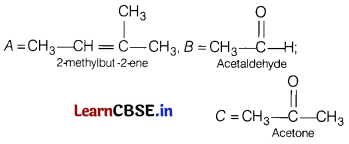
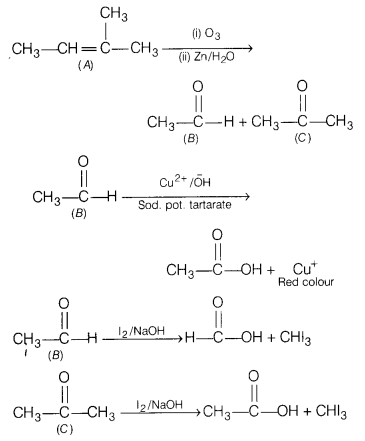
Or
(a) An aromatic compound A (molecular formula C8H8O) gives 2, 4-DNP test. It gives a yellow precipitate of compound B on treatment with iodine and NaOH. A does not gives Tollen’s test. On drastic oxidation alongwith the yellow compound in above reaction.
(i) Identify A, B and C.
(ii) Write equation involved in 2, 4-DNP test of A.
(iii) Write equation of iodoform test of A. [5]
Answer:
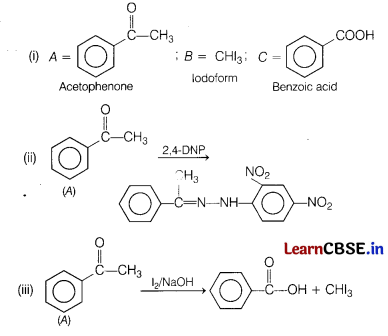
(b) Give the chemical tests to distinguish between
(i) propanol and propanone.
(ii) benzaldehyde and acetophenone.
Answer:
(i) Propanone, because of the presence of —COCH3 group when treated with NaOH and I2, gives yellow crystals of iodoform.
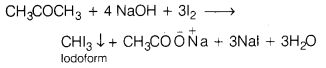
In contrast, propanol (CH3CH2CH2OH) does not contain
group, so it does not give iodoform test.
(ii) Benzaldehyde and acetophenone Both can be distinguished by Tollen’s test. Benzaldehyde contains —CHO group and thus, reduces Tollen’s reagent to metallic silver while acetophenone being a ketone does not reduce Tollen’s reagent.
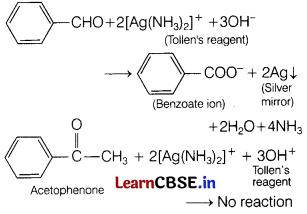
Question 33.
Attempt any five of the following. [5]
(a) Most of the transition metal ions exhibit characteristic colours in aqueous solutions. Why?
Answer:
Most of the complexes of transition elements are coloured. This is because of the absorption of radiation from visible light region to promote an electron from one of the d-orbital to another. These are known as d-d transitions and are responsible for imparting colour to the solution. The ions of transition elements absorb the radiation of a particular wavelength and the rest is reflected, imparting colour to the solution.
(b) Many of the transition elements are known to form interstitial compounds. Explain.
Answer:
In the crystal lattice, transition elements have interstitial vacant spaces into which small sized non-metal atoms such as H, B, C, or N are trapped. These compounds are known as interstitial compounds. These are neither typically ionic nor covalent, e.g. TiC, Mn4N, Fe3H, etc.
(c) Copper (I) ion is not known in aqueous solution. Why?
Answer:
Copper (I) ions are unstable in aqueous solution and undergo disproportionation.
2Cu+ → Cu2+ + Cu
The stability of Cu2+ (aq) rather than Cu+(ag) is due to the much more negative ∆hydH of Cu2+(ag) than Cu+, which compensates more for the second ionisation enthalpy of Cu.
(d) Transition metals and their compounds generally exhibits a paramagnetic behaviour. Give reason.
Answer:
Paramagnetism arises due to the presence of unpaired electrons. When transition metal ions have unpaired electrons in d-orbitals (d1 to d9). They exhibit paramagnetic behaviour.
(e) Cr2+ is a strong reducing agent, whereas Mn3+ with the same configuration is an oxidising agent. Why?
Answer:
Cr2+ is a reducing agent because it can lose an electron to form Cr3+ which has stable 3d3 configuration (as it has half-filled t2g level). On the other hand, Mn3+ can accept an electron to form Mn2+ resulting in the half-filled (d5) configuration which has extra stability. Thus, it behaves as an oxidising agent.
(f) The enthalpies of atomisation of transition metals are quite high.
Answer:
Transition metals have high enthalpies of atomisation due to strong metallic bonding and additional covalent bonding. Metallic bonding is due to their smaller size, while covalent bonding is due to d-d overlapping.
(g) Write two characteristics of the transition elements.
Answer:
The general characteristics at transition element are high melting and boiling point, paramagnetic behaviour, variable oxidation states, catalytic properties, etc.