Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 6 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 6 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
Zero order reaction can be represented graphically as follows
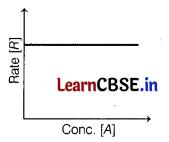
The (i) slope and (ii) rate if the graph are [1]
(a) (i) ln[A] (ii) k
(b) (i) zero (ii) k
(c) (i) -[A] (ii) zero
(d) (i) zero (ii) -[A]
Answer:
(b) (i) zero (ii) k
For zero-order reaction,
Rate = k[A]° = k
The rate does not change with time.
Thus, the slope will be zero and rate = k
Question 2.
The number of ions produced from [Co(NH3)6]Cl3 in solution is [1]
(a) 3
(b) 6
(c) 9
(d) 4
Answer:
(d) 4
Four ions are produced from [Co(NH3)6]Cl3 in solution.
[CO(NH3)6]Cl3 \(\rightleftharpoons\) [Co(NH3)6]+ + 3Cl–
Question 3.
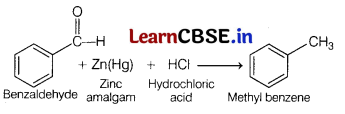
The conversion of benzaldehyde into methylbenzene by zinc-amalgam and hydrochloric acid is called as, [1]
(a) Wolff-Kishner reaction
(b) Rosenmund reaction
(c) Clemmensen reaction
(d) Birch reaction
Answer:
(c) Clemmensen reaction
Clemmensen reaction involves the reduction of aldehyde or ketone to alkane using Zn-Hg and hydrochloric acid.
Question 4.
Which one of the following aldehydes does not give Cannizzaro reaction? [1]
(a) Formaldehyde
(b) Acetaldehyde
(c) Thmethyl acetaldehyde
(d) Benzaldehyde
Answer:
(b) Acetaldehyde
Cannizzaro reaction is given by the aldehydes that do not contain α-H atom. The aldehydes like acetaldehyde, CH3CHO possess α-H atom and hence, does not undergo Cannizzaro reaction.
![]()
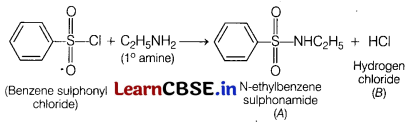
Question 5.
What would be the major product of the following reaction? [1]
C6H5SO2Cl + C2H5NH2 → A + B
(a) A = C6H5SO2NH2, B = C2H5Cl
(b) A = C6H5SO3H, B = C2H4NH2Cl
(c) A = C6H5SO2C2H5, B = NH3
(d) A = C6H5SO2NHC2H5, B = HCl
Answer:
(d) A = C6H5SO2NHC2H5, B = HCl
The reaction of benzene sulphonyl chloride with primary amine yields N-ethyl benzene sulphonamide
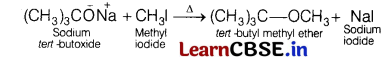
Question 6.
When sodium tert-butoxide and methyl iodide are heated, the major product formed is [1]
(a) dimethyl ether
(b) fert-butyl methyl ether
(c) di-fert-butyl ether
(d) isobutylene
Answer:
(b) tert-butyl methyl ether
Question 7.
Which one of the following compounds will give butanone on oxidation with alkaline KMnO4 solution? [1]
(a) Butan-l-ol
(b) Butan-2-ol
(c) Butene
(d) But-2-ene
Answer:
(b) Butan-2-ol
Butan-2-ol on oxidation with alkaline KMnO4 solution produces butanone. Complete reaction is as follows
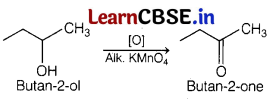
Question 8.
The compound which reacts fastest with Lucas reagent is (at room temperature) [1]
(a) butan-1-ol
(b) butan-2-ol
(c) 2-methylpropan-1-ol
(d) 2-methylpropan-2-ol
Answer:
(d) 2-methylpropan-2-ol
When Lucas reagent is treated with 1°, 2° and 3° alcohols, then turbidity appears. If turbidity is appeared immediately, then alcohol is tertiary. 2-methyl propan-2-ol is a tertiary alcohol. Hence, it reacts fastest with Lucas reagent.
Question 9.
Match the items of Column I and Column II. [1]
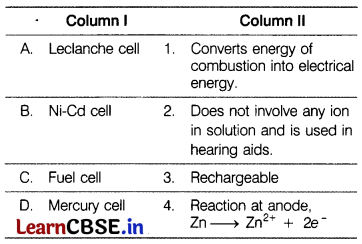
Answer:
(a)
![]()
(a) A – (4),
B – (3),
C – (1),
D – (2).
Question 10.
The coordination number of central metal ion in [Fe(C2O4)3]3- is [1]
(a) 4
(b) 5
(c) 6
(d) 3
Answer:
(c) 6
C2O4 is a bidendate ligand and its value is 3 in the complex. So, the coordination number will be 3 × 2 = 6.
Question 11.
What is the order of a reaction that is 50% complete after 2h and 75% complete after 4h? [1]
(a) First order
(b) Second order
(c) Zero order
(d) None of the above, information given insufficient
Answer:
(a) First order
It is a first order reaction because for 75% completion of reaction two half-lives are required (as, t1/2 = 2h) which suggests that t1/2 is independent of initial concentration.
![]()
Question 12.
Which of the following statement is incorrect? [1]
(a) Ethers are less soluble than corresponding alcohols.
(b) Ethers are polar in nature.
(c) O-atom in ether is sp-hybridised.
(d) Ether has net dipole moment.
Answer:
(c) O-atom in ether is sp-hybridised.
The incorrect statement is (c) because O-atom in ethers show sp3-hybridisation due to the presence of two sigma bond pairs and two lone pair of electrons over it.
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is followed by a corresponding Reason (R) use the following keys to choose the appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R) is true.
Question 13.
Assertion (A) Zero order reactions are relatively uncommon but these occur under special conditions.
Reason (R) Thermal decomposition of HI on gold surface is a zero order reaction. [1]
Answer:
(b) Both (A) and (R) are true, (R) is not the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
Zero order reactions are not so common and they occur under special conditions, like some enzyme catalysed reactions and reactions which occur on metal surface are few examples of zero order reactions.
Moreover, thermal decomposition of HI on gold surface is also zero order reaction.
Question 14.
Assertion (A) Order of basicity of amines in gaseous phase is NH3 > primary amine > secondary amine > tertiary amine.
Reason (R) In gaseous phase, the basicity of amine depends upon the stability of ammonium cation formed by accepting proton from water. [1]
Answer:
(d) (A) is false, (R) is true.
Order of basicity of amines in gaseous phase follows the order
Tertiary amine > secondary amine > primary amine > NH3
The basic nature of aliphatic amines increases with increase in the number of alkyl groups (in gaseous phase). Hence, (A) is false, (R) is true.
Question 15.
Assertion (A) Tollen’s reagent, Benedict’s solution and Fehling solution are reducing agents.
Reason (R) Mild oxidising agents like chlorine or bromine water convert glucose into gluconic acid. [1]
Answer:
(d) (A) is false, (R) is true.
Benedict’s solution, Tollen’s reagent, Fehling’s solution etc., are mild oxidising agents. They oxidise the aldoses and ketoses to the corresponding acids and get themselves reduced. Thus, (A) is false but (R) is true.
Question 16.
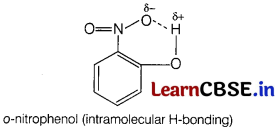
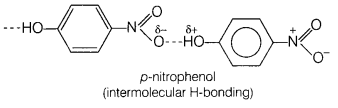
Assertion (A) o-nitrophenol is less volatile than p-nitrophenol.
Reason (R) There is intramolecular hydrogen bonding in o-nitrophenol and intermolecular hydrogen bonding in p-nitrophenol. [1]
Answer:
(d) (A) is false, (R) is true.
(A) is false but (R) is true, o-nitrophenol is more volatile due to intramolecular hydrogen bonding, while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes the association of molecules.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
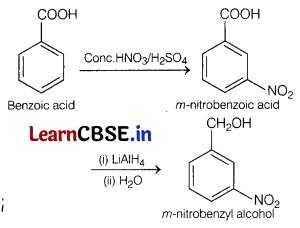
How are the following conversions carried out?
(a) Benzoic acid to m-nitrobenzyl alcohol. [2]
Answer:
Bezoic acid to m-nitrobenzyl alcohol
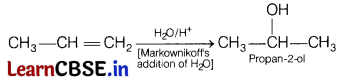
(b) Propene to propan-2-ol.
Answer:
Propene to propan-2-ol
Question 18.
Give reasons for the following.
(a) Measurement of osmotic pressure method is preferred for the determination of molar masses of macromolecules such as proteins and polymers. [2]
Answer:
The osmotic pressure method has the advantage over other methods as pressure measurement is around the room temperature and the molarity of the solution is used instead of molality.
(b) Elevation of boiling point of 1 M KCl solution is nearly double than that of 1 M sugar solution.
Answer:
Elevation in boiling point is directly proportional to ‘i’ ∆Tb ∝ i. Now, as given in the question, elevation of boiling point of 1 M KCI solution is nearly double than that of 1 M sugar solution. It is because KCI being ionic, dissociates into K+ and Cl– and therefore it’s van’t Hoff factor, (i) is 2, whereas for sugar van’t Hoff factor is 1 as it does not undergoes such a dissociation.
Or
(a) Name and state the law for a solution containing volatile components. [2]
Answer:
Raoult’s law For a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Thus, for any component, partial vapour pressure,
p ∝ χ ⇒ p = p°χ
where, p° is the vapour pressure of pure component and χ is the mole fraction of that component.
(b) Out of 1 M glucose and 2 M glucose, which one has a higher boiling point and why?
Answer:
2 M glucose has higher boiling point because more the concentration, more is the elevation in boiling point.
Question 19.
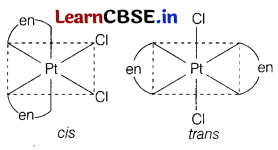
The formula [Pt(en)2Cl2] represent a coordination compound. Write IUPAC name and structures of possible isomers. [2]
Answer:
[Pt(en)2Cl2]
IUPAC name Dichloridob/’s(ethylenediamine)
platinum(II). Geometrical isomers of [Pt(en)2Cl2] are
Question 20.
Explain how and why
(a) aquatic species are more comfortable in cold water than warm water? [2]
Answer:
Oxygen is present in dissolved state in water. As per Henry’s law when temperature rises solubility of a gas decreases in solvent, it means solubility of oxygen in warm water is less than cold water. This makes aquatic species respirate comfortably in cold water.
(b) at higher altitudes, people suffer from anoxia resulting in inability to think?
Answer:
At higher altitude people suffer from anoxia because at higher altitudes, the partial pressure of oxygen is less than that at ground level. This leads to low concentration of oxygen in the blood and tissues of people.
![]()
Question 21.
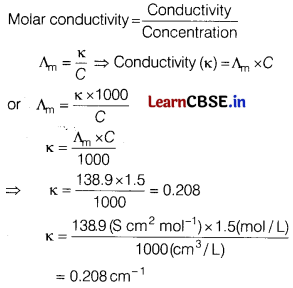
(a) The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2/ mol. What is the conductivity of the solution? [2]
Answer:
Molar conductivity, \(\Lambda_m\) = 138.9 S cm2 mol-1
Molarity = 1.5 M
\(\Lambda_m\) = \(\frac{\kappa \times 1000}{C}\)
\(\kappa\) = \(\frac{\Lambda_{\mathrm{m}} \times C}{1000}\) = \(\frac{138.9 \times 1.5}{1000}\)
\(\mathbf{K}\) = 0.208 cm-1
(b) Why does a salt-bridge is used in a galvanic cell? [1]
Answer:
Salt-bridge connects the solution of two half-cells to complete the cell circuit and it prevents the transference or diffusion of the solutions from one half cell to other.
Section C
(This section contains 7 questions with internal choice in one question. The following questions are
short answer type and carry 3 marks each.)
Question 22.
Write the equations for the following reactions. [3]
(a) Methyl chloride is treated with AgNO2.
Answer:

The reaction between methyl chloride and AgNO2 produces nitromethane and silver chloride. This reaction is an example of substitution reaction.
(b) Bromobenzene is treated with CH3Cl in the presence of anhydrous AlCl3.
Answer:
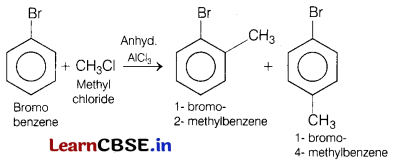
This reaction involves Friedel-Crafts alkylation.
When bromobenzene is treated with CH3Cl in the presence of anhydrous AlCl3, it gives 1 -bromo-2-methylbenzene and-1 -bromo-4-methyl benzene as major product.
(c) Ethyl chloride is treated with aqueous KOH.
Answer:
Ethyl chloride undergoes hydrolysis to form ethyl alcohol (through SN2 nucleophilic substitution).

Question 23.
(a) Identify the product formed when propene undergoes anti-Markowinikoff’s addition. Name the reagent which is used to carry out the reaction. [3]
Answer:
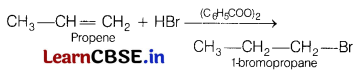
This reaction is an example of anti-Markownikoff’s addition in which negative part of hydrohalide goes to that carbon which is having more number of hydrogen in the presence of peroxide.
(b) Why do haloalkanes easily dissolve in organic solvents?
Answer:
Haloalkanes easily dissolve in organic solvents because both are covalent in nature (like dissolve like). Also the steric effect in haloalkanes is not more as seen in the case of haloarenes.
Or
(a) Name the product formed when p-nitrochlorobenzene is heated with aqueous NaOH at 443 K, followed by acidification. Write the reactions involved.
Answer:
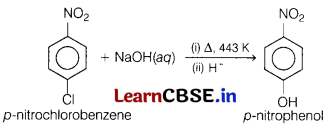
In this reaction, p-nitrochlorobenzene is converted into p-nitrophenol when reacted with NaOH in the presence of heat followed by acidification.
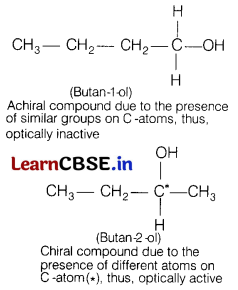
(b) Butan-1-ol is optically inactive but butan-2-ol is optically active. Why?
Answer:
Butan-1 -ol is optically inactive compound due to the absence of asymmetric C-atom (or chiral C-atom) whereas butan-2-ol is optically active compound due to the presence of asymmetric C-atom (or chiral C-atom).
Question 24.
Give reasons of the following observations.
(a) Gabriel phthalimide synthesis is not preferred for synthesising aromatic primary amines. [3]
Answer:
Aryl halides do not undergo nucleophilic substitution with salt formed by phthalimide. That’s why Gabriel phthalimide synthesis is not preferred to prepare aromatic primary amines.
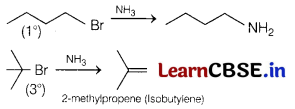
(b) Tert-butylamine cannot be prepared by the action of NH3 on tert-butyl bromide.
Answer:
Ammonolysis (action of NH3) gives the highest yield with 1° halides where substitution predominates because 10 halides behave as nucleophile While in case of 3° halide elimination predominates where 3° halide acts as base.
Therefore, tert-butylamine cannot be prepared by the action of NH3 on tert-butyl bromide.
(c) Acetylation of aniline reduces its activation effect.
Answer:
Due to electron withdrawing effect of the acetyl group, the lone pair of electrons on N-atom is attracted by acetyl group.
As a result, lone pair of electrons on N-atom is not exclusively available for donation to the benzene ring and hence, activating effect of the —NH2 group is reduced.
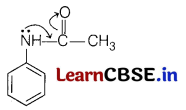
Question 25.
How are vitamins classified? Name the vitamin responsible for the coagulation of blood. [3]
Answer:
Classification of vitamins Vitamins are classified into two groups depending upon their solubility in water or fat
- Fat soluble vitamins
- Water soluble vitamins
Vitamin K is responsible for the coagulation of blood.
Question 26.
Using crystal field theory, draw energy level diagram, write electronic configuration of central atom/ion and determine the magnetic moment. [3]
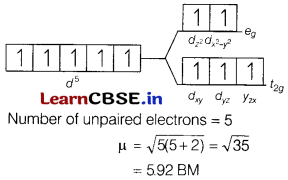
(a) [FeF6]3-
Answer:
The electronic configuration of Fe3+ is [Ar]3d5. Since F– is a weak field ligand, ∆0 < P. Therefore, a high spin complex is formed. The orbital diagram of [FeF6]3- is as follows
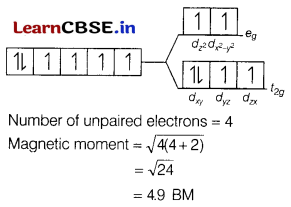
(b) [Fe(H2O)6]2+
Answer:
[Fe(H2O)6]2+
The electronic configuration of Fe2+ is [Ar]3d6.
Since, H2O is also a weak field ligand, ∆0 < P. Therefore, a high spin complex is formed. The orbital diagram of Fe(H2O)2+ is as follows
(c) [Fe(CN)6]4-
Answer:
[Fe(CN)6]4-
The electronic configuration of Fe2+ is [Ar]3d6. But CN– is strong field ligand. Thus, pairing of electron will takes place, ∆0 > P. Therefore, a low spin complex is formed. The orbital diagram of [Fe(CN)6]4- s as follows
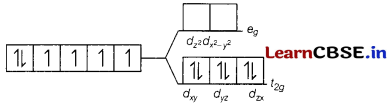
No unpaired electrons, so diamagnetic.
Magnetic moment = 0
Question 27.
Answer the following questions. [3]
(a) Henry’s law is for a solution containing volatile components. Then how does Raoult’s law become a special case of Henry’s law?
Answer:
In the solution of a gas in a liquid, one of the components is so volatile that it exists as a gas and its solubility is given by Henry’s law which states that,
P = KHχ.
i.e. partial pressure of the volatile component (gas) is directly proportional to the mole fraction of that component (gas) in the solution.
When the equations of Raoult’s law and Henry’s law are compared, it can be seen that the partial pressure of the volatile component or gas is directly proportional to its mole fraction in solution. Only the proportionality constant KH differs from p°.
(b) Assume that N2 exerts a partial pressure of 0.987 bar. If N2 gas is bubbled through water at 293 K, then what will be its mole fraction? Given that Henry’s law constant for N2 at 293 K is 76.48 kbar.
Answer:
According to Henry’s law,
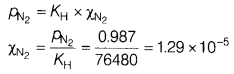
Question 28.
Amino acids may be acidic, alkaline or neutral. How does this happen? What are essential and non-essential amino acids? Name one of each type. [3]
Answer:
Amino acids may be acidic, basic or neutral depending upon the relative number of amino and carboxyl group present in the molecule.
Equal number of amino and carboxyl groups makes it neutral.
More number of amino groups than carboxyl groups makes it basic and more carboxyl groups as compared to amino groups makes it acidic.
- Essential amino acids are the a-amino acids which are needed for health and growth of human beings but are not synthesised by the human body, e.g. valine, leucine.
- Non-essential amino acids are the a-amino acids which are needed for health and growth of human beings and are synthesised by the human body, e.g. glycine, aspartic acid.
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
Within the 3d-series, manganese exhibits oxidation states in aqueous solution from +2 to +7. Likewise, iron forms both Fe2+(ag) and Fe3+(ag). Cr and Mn form oxy-ions Cr\(\mathrm{O}_4^{2-}\), Mn\(\mathrm{O}_4^{-}\), owing to their willingness to form multiple bonds.
The pattern with the early transition metals in the 3d series up to Mn, and for the 4d, 5d metals up to Ru and Os is that the maximum oxidation state corresponds to the number of “outer shell” electrons.
The highest oxidation states of 3d metals may depend upon the complex formation (e.g. the stabilisation of Co3+ by ammonia) or upon the pH (thus Mn\(\mathrm{O}_4^{2-}\)(ag) is prone to disproportionation in acidic solution). Within the 3d-series, there is considerable variation in relative stability of oxidation states, sometimes on moving from one metal to a neighbour; thus, for iron, Fe3+ is more stable than Fe2+, especially in alkaline conditions, while the reverse is true for cobalt.
The ability of transition metals to exhibit a wide range of oxidation states is marked with metals such as vanadium, where the standard potentials can be rather small, making a switch between states relatively easy.
Answer the following questions. [4]
(a) In 3d-series, Mn exhibits highest oxidation state while in 4d and 5d, Ru and Os has the highest oxidation state. What is the highest oxidation state among the given elements?
Answer:
Ru and Os exhibit highest state in their respective series. Both shows + 8 oxidation state (Mn show) +7.
Or
Although zirconium belongs to 4 d-transition series and hafnium to 5d-transition series even then they show similar physical and chemical propeties. Give reason. [4]
(b) Vanadium has the ability to exhibit various oxidation states. How do you explain this?
(c) Complete and balance the following equations.
(i) Fe2+ + Mn\(\mathrm{O}_4^{-}\) + H+ →
(ii) Mn\(\mathrm{O}_4^{-}\) + H2O + I– →
Answer:
Due to lanthanoid contraction, Zr and Hf possess nearly same atomic and ionic radii, i.e. atomic radii are Zr = 160 pm and Hf = 159 pm and ionic radii are Zr4+ = 79 pm and Hf4+ = 78 pm. Therefore, these two elements show similar chemical and physical properties.
(b) Vanadium has the ability to exhibit wide range of oxidation states from + 2 to + 5 as it has 3d3 4s2 outer electronic configuration and its standard
potentials are rather small due to stability of V2+ as it has half-filled t2g level.
(c)

Question 30.
For a reaction, 2A + B → 2C following data were calculated experimentally. The experiments were performed with varied concentration and for each initial rate of formation of C was recorded. [4]
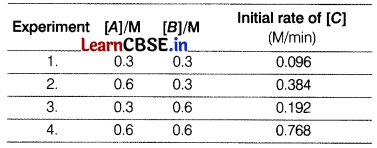
Answer the following questions on the basis of above data.
(a) Why were the experiments performed multiple times? Justify.
Answer:
Different sets of data help in avoiding error in data collection and provide more accurate data for analysis.
(b) Calculate the order of the reaction.
Answer:

(c) (i) Write the rate law of the reaction.
(ii) Which experiment is the fastest in term of +he data? Justify.
Answer:
(i) Rate law of the reaction, R = k[A]2[B]1
(ii) Experiment 4 is the fastest as in 1 minute, 0.768 molar concentration of [C] was formed.
Or
Calculate rate constant of the reaction. Justify it. [4]
Answer:
Rate constant k.
From Exp. 1,
Rate = k[A]2[B]1
0.096 = k (0.3)2 (0.3)1
k = \(\frac{0.096}{0.3 \times 0.3 \times 0.3}\) = \(\frac{96}{27}\)
= 3.55 m-2 min-1
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
Attempt any five of the following. [5]
(a) Why is the E°value for Mn3+ /Mn2+ couple is much more positive than that for Fe3+ /Fe2+?
Answer:
Mn2+ compounds are more stable due to half-filled d-orbitals. Fe2+ compounds are comparatively less stable as they have six electrons in their orbitals.
So, they tend to lose one electron from Fe2+ and get stable 3d 5 configuration in Fe3+.
Therefore, comparatively high positive value of E° for Mn3+ /Mn2+ indicates the stability of Mn2+(d5) whereas comparatively low value for Fe3+ / Fe2+ indicates the extra stability of Fe3+ (d5).
(b) Why transition metals form alloys?
Answer:
Atoms of transition metal can easily take place in the crystal lattice of another metal in the molten state and are miscible with each other and thus forms alloys.
(c) Why Eu2+ is a strong reducing agent?
Answer:
Eu2+ having electronic configuration [Xe]4f75d16s0 is a strong reducing agent because in the aqueous solution, it gets back to its most stable +3oxidation state which has [Xe]4f76d06s0 stable electronic configuration.
(d) Explain why transition elements acts as catalyst?
Answer:
Many transition metals and their compounds are used as catalyst.
Their catalytic activity is due to their ability to adopt multiple oxidation states and to form complexes. Transition metals acts as catalyst because of their variable valencies sometimes form unstable intermediate compounds and provide a new path with lower activation energy for the reaction, e.g. V2O5 (contact process), finely divided iron (in Flaber’s process) and nickel (in catalytic hydrogenation).
(e) What is the lanthanoid contraction?
Answer:
Lanthanoid contraction The overall decrease in atomic and ionic radii from lanthanum to lutetium, due to the imperfect shielding of one electron by another in the same subshell is known as lanthanoid contraction.
(f) Why Zn2+ salts are white, while Cu2+ salts are coloured?
Answer:
Cu2+ salts are coloured because Cu2+ ion has 3d94s0 valence shell configuration with one unpaired electron and therefore, it is paramagnetic in nature.
Hence, Cu2+ ion undergoes d-d transition and forms coloured salts.
In contrast to Cu2+, Zn2+ has all paired electrons due to 3d104s0 valence shell configuration and therefore, it does not undergo d-d transition. Flence, Zn2+ salts are white.
(g) Why transition elements show variable oxidation states?
Answer:
ns and (n -1 )d electrons of transition metal have very little difference in the energies and hence both can participate in bonding, which results in variable oxidation states.
When ns-electrons take part in bonding, they exhibit lower oxidation states, whereas when (n – 1)d-electrons alongwith ns-electrons participate in bonding, they exhibit variable oxidation states.
Question 32.
(a) Write Nernst equation for the following cell reaction, [5]
Zn |Zn2+(ag) || Cu2+(ag) | Cu(s)
Answer:
Nernst equation of given cell is
Ecell = E°cell – \(\frac{2.303 R 7}{2 F}\) = log\(\frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}\)
(b) Write the equations for the reactions taking place at the two electrodes during the electrolysis of acidified copper sulphate solution with copper electrodes.
Answer:
At anode, Cu → At cathode, Cu2+ + 2e–
At cathode, Cu2+ + 2e– → Cu
Here, both copper electrodes are used.
(c) What is the effect of increase in concentration of zinc ions on the electrode potential of zinc electrode for which \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}\) equals to – 0.76 V?
Answer:
According to Nernst equation,
![]()
Hence, electrode potential will increase with increase in concentration of Zn+ ions.
Or
(a) The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2/mol. What is the conductivity of the solution?
Answer:
Molar conductivity, Am = 138.9 S cm2 mol
Molarity = 1.5 M, Conductivity, \(\kappa\) = ?
(b) What is the standard free energy change for the following reaction at room temperature? Is the reaction spontaneous?
Zn(s) + Cu2+(ag) → Zn2+(aq) + Cu(s)
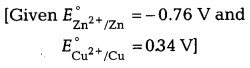
Answer:
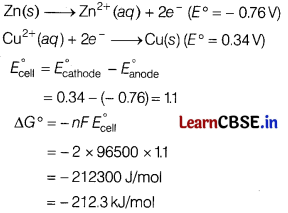
As the value of ∆G° is negative, so it is a spontaneous reaction.
![]()
Question 33.
Compound A (C6H12O2) on reduction with LiAlH4 yielded two compounds B and C. The compound B on oxidation gave D which on treatment with aqueous alkali and subsequent heat produces E. Also, the oxidation reaction of D gave monobasic acid (molecular weight 60) and the catalytic hydrogenation of E gives the compound C. Identify A, B, C, D and E. [5]
Answer:
The compound A which gives CH3CH2OH on reduction with LiAIH4 will be CH3COOC4H9 (n-butyl acetate).
Since, the compound D has been obtained by oxidation of the compound B. Therefore, B is an alcohol having formula CH3CH2OH.
The compound E is monobasic acid having molecular weight 60. Therefore, it is acetic acid CH3COOH.
The compound D which on oxidation gives acetic acid is CH3CHO.
The reactions involved may be given as

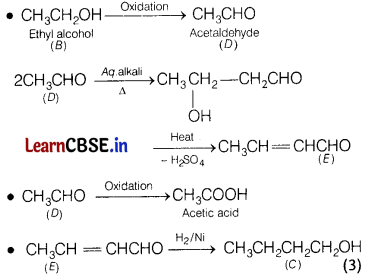
Or
(a) Give reasons of the following observations. [5]
(i) CH3CHO is more reactive than CH3COCH3 towards reaction with HCN.
Answer:
CH3CHO is more reaction than CH3COCH3 towards reaction with HCN because of the fact that due to smaller +I-effect of one alkyl group (—CH3) in CH3CHO as compared to larger +I-effect of two alkyl (—CH3)2 groups in CH3COCH3, the magnitude of positive charge on the carbonyl carbon is more in CH3CHO than in CH3COCH3.
Also, the steric effect is more pronounced in case of CH3COCH3.
(ii) Cl—CH2COOH is a stronger acid than CH3COOH.
Answer:
CI —CH2COOH is a stronger acid than CH3COOH. It is because —CI group exhibits -I-effect which makes the carboxylate ion more stable.
Higher the stability of carboxylate ion, easier is the removal of proton from the carboxylic acid and stronger is the acid. In CH3COOH, —CH3 group has + I-effect which destabilises it. Hence, CH3COOH is a weaker acid.
(iii) Aromatic carboxylic acids do not undergo Friedel-Crafts reaction.
Answer:
Due to the presence of strong —COOH group, aromatic carboxylic acids do not undergo for Friedel-Crafts reaction.
(b) Arrange the following compounds in the increasing order of their property as indicated.
(i) CH3COCH3, C6H5COH3, CH3CHO (reactivity towards nucleophilic addition reaction)
Answer:
The increasing order of their reactivity towards nucleophilic addition reaction is
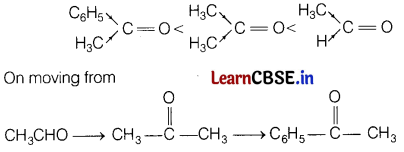
+I -effect, i.e. electron donating effect of alkyl group increases which increases the electron density on C-atom of carbonyl group, and inC6H5COCH3, phenyl group get resonance stabilised, making it stable.
Due to this reason it is less reactive towards nucleophilic addition as the attack of nucleophile becomes lower.
Further, steric effects of methyl and phenyl groups around carbonyl carbon atom makes the attack of nucleophile on carbonyl carbon difficult.
(ii) ClCH2COOH, FCH2COOH, CH3COOH (acidic character)
Answer:
The correct increasing order of their acidic character is
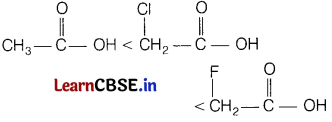
F-being more electronegative element produces greater-I-effect than Cl-atom due to which F-atom withdraw electrons from O—H bond and thereby making O—H bond weaker and hence, facilitates the release of H+ ion from O—H bond.
Hence, FCH2COOH is stronger acid than CICH2COOH and CH3COOH.
In CH3COOH, due to +I-effect of methyl group, electron density in O—H bond increases. As a result, release of H+ ions from acetic acid becomes more difficult.