Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 4 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 4 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
Out of o-chlorophenol, o-bromophenol, o-iodophenol and o-fluorophenol, which is most acidic ? [1]
(a) o-bromophenol
(b) o-chlorophenol
(c) o-iodophenol
(d) o-fluorophenol
Answer:
(b) o-chlorophenol
o-chlorophenol will be most acidic. Here, o-fluorophenol is weakest acid due to strong intramolecular H-bonding. The acidity of other halophenol decreases as the -I-effect of halogen decreases.
Question 2.
Which of the following alkyl halides is hydrolysed by SN2 mechanism? [1]
(a) C6H5CH2Br
(b) CH3Br
(c) CH2 = CHCH2Br
(d) (CH3)3CBr
Answer:
(b) CH3Br
SN2 mechanism involves the back side attack and formation of a transition state, thus less hindered alkyl halide readily undergoes SN2 mechanism. Among the given haloalkanes, CH3B (methyl bromide) is less hindered, thus hydrolysed by SN2 mechanism.
Question 3.
Consider the following graph,
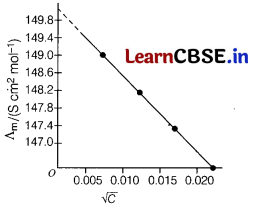
Here, the limiting molar conductivity is [1]
(a) 148.6
(b) 150
(c) 87.46
(d) 147
Answer:
(b) 150
When concentration approaches zero, the molar conductivity is known as limiting molar conductivity. So, here limiting molar conductivity is near about 150.
![]()
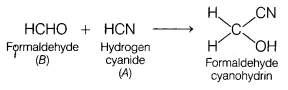
Question 4.
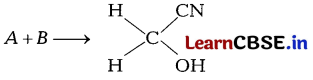
Identify A and B [1]
(a) A = Acetone , B = Methyl cyanide
(b) A = Hydrogen cyanide, B = Formaldehyde
(c) A = Acetonitrile, B = Ethanal
(d) A = Methanol, B – Methyl cyanide
Answer:
(b) A = Hydrogen cyanide, B = Formaldehyde
Question 5.
Glycogen is a polymer of α-D-glucose stored in the [1]
(a) liver, kidney and brain of animals.
(b) kidney, lungs and stomach of animals.
(c) brain, liver and muscles of animals.
(d) stomach, muscles and lungs of animals.
Answer:
(c) brain, liver and muscles of animals.
Glycogen is a polymer of a-D-glucose stored in the liver, brain and muscles of animals, also known as animal starch.
Question 6.
Match the magnetic moment with the ions of 3d-series. [1]
| (i) Cr2+ | (p) 5.92 BM |
| (ii) Mn2+ | (q) 3.87 BM |
| (iii) Fe2+ | (r) 4.90 BM |
| (s) zero |
(a) (i)-(r), (ii)-(q), (iii)-(p)
(b) (i)-(r), (ii)-(s), (iii)-(p)
(c) (i)-(q), (ii)-(p), (iii)-(r)
(s) (i)-(s), (ii)-(r), (iii)-(p)
Answer:
(c) (i)-(q), (ii)-(p), (iii)-(r)
The number of unpaired electrons in Cr3+, Mn2+ and Fe2+ are 3, 5 and 4 respectively. By using formula for magnetic moment, i.e. µ = \(\sqrt{n(n+2)}\) where, n is number of unpaired electrons, the magnetic moment of Cr3+, Mn2+ and Fe2+ comes to be 3.87 BM, 5.92 BM and 4.90 BM respectively.
Question 7.
For the reaction, A → P, the rate of reaction increases eight times when the concentration of the reactant increases four times. The order of the reaction is [1]
(a) 2.5
(b) 0.5
(c) 1.5
(d) 2.0
Answer:
(c) 1.5
For the reaction, A → P
The differential rate law is
Rate = k[A]x
Rate1 = k[a]x
Rate2 = k[4a]x
Thus, \(\frac{\text { Rate }_2}{\text { Rate }_1}\) = \(\left(\frac{4 a}{a}\right)^x\)
⇒ 8 = (4)x [∵ Rate2 = 8 Rate]
Taking log both sides
log 8 = log(4)x
⇒ log(2)3 = log(2)2x
⇒ 3log2 = 2 × log2
⇒ x = \(\frac{3}{2}\) = 1.5
∴ Hence, order of the reaction is 1.5.
Question 8.
Which of the following statements is not correct for amines? [1]
(a) The angle of C—N—C in trimethylamine is 108°.
(b) Me—NH2 is the weaker base than MeOH.
(c) The amine formed has one carbon atom less than the parent 1° amide in Hofmann bromamide reaction.
(d) N-ethyl benzene sulphonamide is soluble in alkaline medium.
Answer:
(b) Me—NH2 is the weaker base than MeOH.
MeNH2 is a stronger base than MeOH because N is less electronegative than 0 and lone pair of electrons on N is more easily available for the donation in MeNH2.
Question 9.
What would be the product of the following reaction? [1]
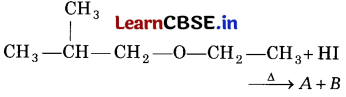
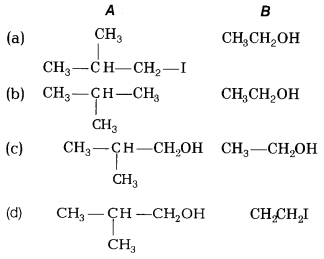
Answer:
(d)
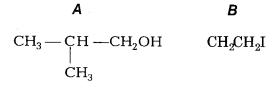
The reaction of iso-propyl ethyl ether with hot hydroiodic acid gives iso-propyl alcohol (A) and ethyl iodide (B).
Question 10.
The half-life for a first order reaction is 240 s, the time after which 99.9% reaction gets completed is [1]
(a) 16 min
(b) 8 min
(c) 32 min
(d) 40 min
Answer:
(d) 40 min
Given, half-life for a first order reaction (t1/2) = 240 s = 4 min.
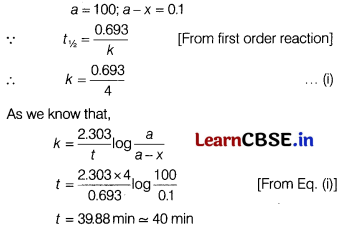
Question 11.
CH3CH2OH can be converted into CH3CHO by [1]
(a) catalytic hydrogenation
(b) treatment with LiAlH4
(c) treatment with pyridinium chlorochromate
(d) treatment with KMnO4
Answer:
(c) treatment with pyridinium chlorochromate
Ethanal is an oxidised product of ethanol.
Pyridinium chlorochromate \(\left(\mathrm{PCC}=\mathrm{C}_6 \mathrm{H}_5 \stackrel{+}{\mathrm{N}} \mathrm{H} \stackrel{-}{\mathrm{C}} \mathrm{ICrO}_3\right)\) oxidises primary alcohols to aldehydes. It stops reaction at the aldehydic stage.
Strong oxidising agents such as KMnO4 are used for getting carboxylic acids from alcohols.
![]()
Question 12.
Lanthanoides form complex with which of the following ligand? [1]
(a) F–
(b) Br–
(c) Cl–
(d) None of these
Answer:
(a) F–
F– have small size and high electronegativity thus, it forms complex with lanthanides easily.
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is followed by a corresponding Reason (R). Use the following keys to choice the appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R) is true.
![]()
Question 13.
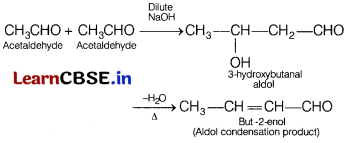
Assertion (A) Acetaldehyde on reaction with dil. NaOH forms aldol.
Reason (R) Aldehydes and ketones having α-hydrogen undergo aldol condensation. [1]
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A). Acetaldehyde has 3 α- hydrogen atoms, so can easily undergo aldol condensation reaction.
Question 14.
Assertion (A) Electrolysis of NaCl solution gives chlorine at anode instead of O2.
Reason (R) Formation of oxygen at anode requires over voltage. [1]
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
During the electrolysis of NaCl, the following reactions take place.
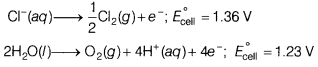
The reaction with lower value of E° preferred, and therefore water should get oxidised in preference to Cl–(aq). However, due to overvoltage, chlorine is liberated at anode.
Question 15.
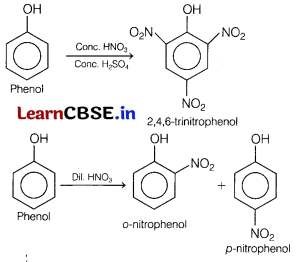
Assertion (A) Phenols give 0- and p-nitrophenol on nitration with cone. HNO3 and H2SO4 mixture.
Reason (R) —OH group in phenol is o – and p-directing. [1]
Answer:
(d) (A) is false but (R) is true.
Phenols giveo- and p-nitrophenol on nitration with dil. HNO3 at 298 K.
Question 16.
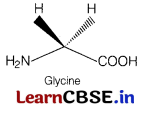
Assertion (A) All naturally occurring α-amino acids except glycine are optically active.
Reason (R) Most naturally occurring amino acids have L-configuration. [1]
Answer:
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A). All naturally occurring a-amino acids except glycine are optically active.
Glycine is optically inactive because glycine does not have all four different substituent as shown below.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
(a) What is the difference between molarity and molality of a solution? [2]
(b) A solution of glucose (C6H12O6) in water is labelled as 10% by weight. What would be the molality of the solution? (Molar mass of glucose = 180 g mol-1).
Answer:
(a) Molality is defined as the number of moles of the solute present in per kilogram of the solvent. It is represented by m. It does not change with change in temperature.
Molarity is defined as the number of moles of solute dissolved in one litre or one cubic decimetre of the solution.
It decreases with increase in temperature (as V ∝ T).
(b) A 10% glucose solution by weight means that 10 g glucose is present in 100 g solution.
Number of moles of 10 g glucose
= \(\frac{10}{180}\) = 0.0555 mol
Weight of glucose = 10 g
Weight of water = 90 g = \(\frac{90}{1000}\)kg = 0.09 kg
Molality (m) \(=\frac{\text { Number of moles of solute }}{\text { Mass of solvent (in kg) }}\)
= \(\frac{0.0555 \mathrm{~mol}}{0.09 \mathrm{~kg}}\)
= 0.61 mol kg-1 or 0.61 m
Question 18.
A first order reaction is 25% complete in 40 minutes. Calculate the value of rate constant. In what time will the reaction be 80% completed?
(Given : log 3 = 0.477, log 4 = 0.602) [2]
Answer:
For first order reaction, k = \(\frac{2.30}{t}\) log \(\frac{a}{a-x}\)
The given first order reaction completes 25% in 40 min.
Let, a = 100
a – x = 100 – 25,
a – x = 75
∴ k = \(\frac{2.303}{40}\)log\(\frac{100}{75}\)
= \(\frac{2.303}{40}\)(log 4 – log 3)
k = 0.0072 min-1
For 80% completion of reaction,
a = 100
a – x = 100 – 80 = 20
k = 0.0072 min-1
t = \(\frac{2.303}{0.0072}\)log\(\frac{100}{20}\)
= 223.6 min
Question 19.
(a) Explain why cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethyl cyclohexanone does not.
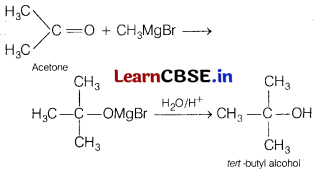
(b) How will you obtain tertiary butyl alcohol from acetone? [2]
Answer:
(a)
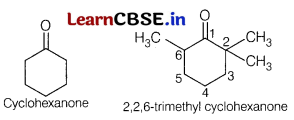
In 2,2,6-trimethyl cyclohexanone, three methyl groups are presents at a-position with respect to the ketonic \((>C=0)\) group. Therefore, these groups cause steric hindrance during the nucleophilic attack of CN– ion and cyanohydrin is not formed. Due to the absence of methyl groups in cyclohexanone, there is no steric hindrance and cyanohydrin is formed.
(b)
Question 20.
Give reason for the following.
(a) t-butyl bromide is more reactive towards SN1 reaction as compared to n-butyl bromide.
(b) CH3CH2I undergoes SN2 reaction faster than CH3CH2Br. [2]
Or
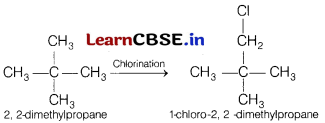
(a) A hydrocarbon C5H12 gives only one monochlorination product. Identify the hydrocarbon.
(b)
Out of
![]()
and
![]()
which one undergoes faster towards SN1 reaction? [2]
Answer:
(a) In SN1 reaction, reactivity depends upon the stability of intermediate carbocation formed. Let us consider the formation of carbocation of the two given alkyl halides.
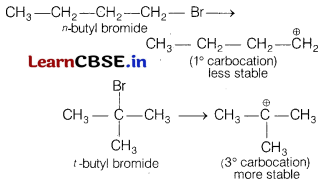
Carbocation formed by tert-butyl bromide being tertiary is more stable than primary, hence t-butyl bromide undergoes SN1 reaction faster.
(b) As I– ion is a better leaving group than Br ion, therefore iodides are more reactive than bromides. Therefore, CH3—CH2—I is more reactive than CH3—CH2—Br towards SN2 reaction and thereby, CH3—CH2—I would undergo SN2 reaction faster than CH3—CH2—Br.
Or
(a) Hydrocarbon which gives only one
monochlorination product is 2, 2-dimethylpropane.
(b) The reactivity towards SN1 reaction depends upon the stability of the intermediate carbocation which an alkyl halide gives on ionisation. Thus,
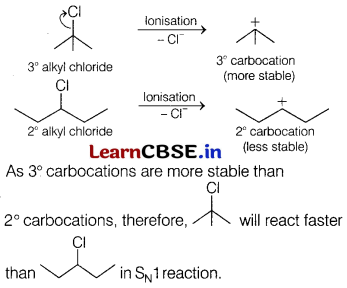
Question 21.
(a) Amino acids show amphoteric behaviour. Justify your answer.
(b) Explain what is meant by pyranose structure of glucose? [2]
Answer:
(a) Amino acids contain both amino (—NH2) and carboxyl (—COOH) groups, thus they react with both acids and bases. Hence, amino acids are amphoteric in nature.
(b) The six membered cyclic structure of glucose is called pyranose structure (α or β), in analogy with pyran. Pyran is a cyclic compound with one oxygen atom and five carbon atoms in the ring.
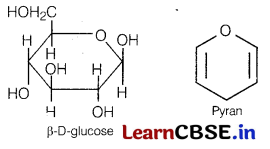
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
(a) By giving an example, explain the use of complex compounds in qualitative analysis.
(b) Write the formula for the following coordination compound.
Iron (III) hexacyanidoferrate (II)
(c) Explain why are low spin tetrahedral complexes not formed. [3]
Answer:
(a) EDTA reagent forms various coloured hybrid compounds with many metal ions (such as Ca2+, Ca, Zn2+, Fe2+, CO2+, Ni2+ etc.) which are used to identify these metal ions. (1)
(b) Fe4[Fe(CN)6]3.
(c) For tetrahedral complexes, the crystal field stabilisation energy is lower than pairing energy, so they are not formed in low spin state.
Question 23.
(a) Explain strong and weak electrolytes with examples.
(b) Calculate the emf of the cell.
Mg(s)|Mg2+ (0.1M) | | Cu2+ (1 × 10-1M) |Cu(s)
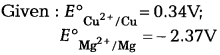
(c) Arrange the following metals in the order in which they displace each other from the solution of their salts Al, Cu, Fe, Mg and Zn. [3]
Answer:
(a) Strong electrolytes These electrolytes are those completely dissociate into ions at all concentrations, e.g. NaOH, NaCl, KCl etc.
Weak electrolytes The electrolytes which do not ionise completely in aqueous as well as in molten state are called weak electrolytes, e.g. H2CO3, HgCl2, CH3COOH etc.
(b)

(c) Mg > Al > Zn > Fe > Cu
![]()
Question 24.
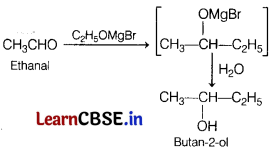
Write the reaction and IUPAC name of the product formed.
(a) When ethanal is treated with ethyl magnesium bromide followed by hydrolysis.
(b) Phenol is treated with conc. H2SO4 and conc. HNO3. [3]
Answer:
(a) When ethanol is treated with ethyl magnesium bromide, then unstable compound is formed which further on hydrolysis, gives final product, i.e. butan-2-ol.
(b) When phenol is treated with conc. H2SO4 and cone. HNO3, the nitration of phenol takes place and 2, 4, 6-trinitrophenol is obtained.
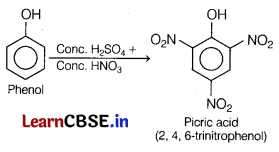
Question 25.
An organic compound A (C4H6O4) on treatment with ethyl alcohol gives a carboxylic acid B and compound C. Hydrolysis of C under acidified conditions gives B and D.
Identify A, B, C and D and write all the involved equations. [3]
Or
A ketone ‘A’ (C4H8O) which undergoes a haloform reaction gives compound B on reduction, heating with sulphuric acid gives a compound C which forms mono-ozonides D. Identify A, B, C and D and write all the involved equations. [3]
Answer:
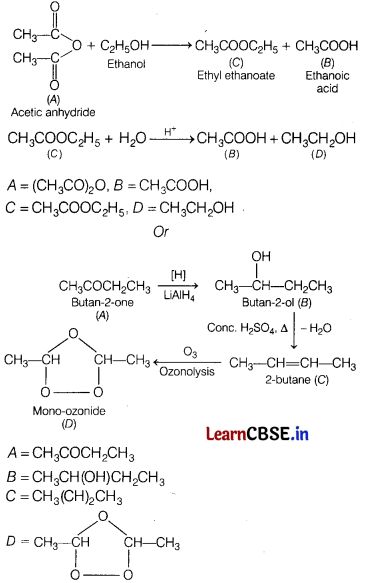
Question 26.
The three solutions of fructose, sucrose and glucose are prepared in 10% water at 2d°C. The optical rotation values are measured by the student using a polarimeter and are given in the following table. [3]
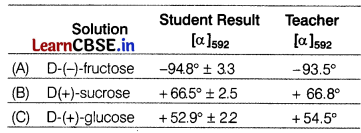
(a) What does the positive and negative optical rotation in the above results indicate?
(b) There can be a change in the sign of rotation from dextro to laevo in a reaction. Justify your answer with example.
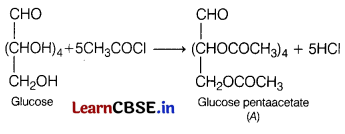
(c) If glucose reacts with acetyl chloride or acetic anhydride, then compound A is obtained. Identify the compound A.
Answer:
(a) For clockwise direction, the optical rotation (in degrees) is defined as positive and called dextrorotatory. In contrast, the counterclockwise direction is defined as negative and called laevorotatory.
(b) When hydrolysis of sucrose takes place, there is a change in the sign of rotation from dextro to laevo, because of the change in optical rotation.
(c) The reaction of glucose with acetyl chloride or acetic anhydride gives glucose pentaacetate, i.e. compound A.
Question 27.
Name the possible alkene which will yield toluene on their reaction with sodium and methyl chloride. Write the name of reaction involved and its mechanism also. [3]
Answer:
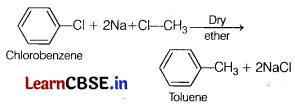
Chlorobenzene reacts with sodium and methyl chloride in the presence of dry ether to form toluene and sodium chloride. This reaction is known as Wurtz reaction.
Mechanism involved :
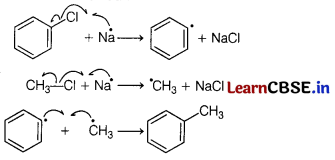
Question 28.
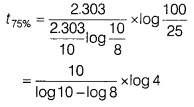
A first order reaction is 20% complete in 10 minutes. Find the time required for 75% completion of the reaction. [3]
Answer:
For the first order reaction,

By substituting value of k from Eq. (i) in Eq. (ii),
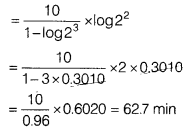
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
Relationship between Wavelength and Colour of Coordination Compounds
The colour of light is determined by the amount of valence electrons present in a compound’s outermost orbit. These electrons absorb a certain wavelength of visible light and emit a colour that is complementary to the wavelength absorbed. The colour for a coordination complex can be predicted using the Crystal Field Theory (CFT). The table given below shows the different wavelength absorbed by the coloured complexes.
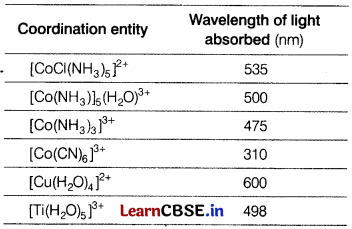
Answer the following questions. [4]
(a) Among the given complexes, which have the strong held ligand and give the IUPAC name of that complex?
Or
Out of the ligands attached to the given complexes, which will cause maximum splitting and why?
(b) Which of the given compounds is pale yellow in colour ?
(c) What is spectrochemical series and arrange the mentioned ligands (in the table) according to spectrochemical series?
Answer:
(a) \(\mathrm{CN}^{-} \text {in }\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}\) is a strong field ligand and IUPAC name of this complex is hexacyanidocobaltate(III).
Or
As CN– is a strong field ligand, hence it will cause maximum splitting. [4]
(b) [Co(CN)6]3- is the most stable complex, thus it absorbs light of minimum wavelength. Hence, it has lightest colour, i.e. pale yellow.
(c) Spectrochemical series is the series which gives the arrangement of ligands in the increasing order of crystal field splitting.
The order from weak to strong ligands according to spectrochemical series is
Cl– < H2O < NH3 < CN.
Question 30.
A knowledge of the electrode potential is of utmost importance in order to design any electrochemical device or to carry out any meaningful measurement. The reactions carried out electrochemically can be energy efficient and less polluting. Therefore, study of electrochemistry is important for creating new technologies that are ecofriendly. To know about the electrochemistry, the related terms should be known. A potential difference develops between the electrode and the electrolyte which is called electrode potential. When the concentrations of all the species involved in a half-cell is unity then the electrode potential is known as standard electrode potential.
The redox reaction that occurs during an electrochemical process.The oxidation-reduction reactions take place in two half-reactions, one representing the oxidation process and’one the reduction process. The sum of the half-reactions gives the overall chemical reaction. Single electrode potential cannot be determined. An electric current is produced from the flow of electrons from the reductant to the oxidant. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a non-spontaneous reaction. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is consumed to drive a non-spontaneous redox reaction. Both types of cells use two electrodes that provide an electrical connection between systems that are separated in space.
The oxidative half-reaction occurs at the anode, and the reductive half-reaction occurs at the cathode. A salt-bridge connects the separated solutions, allowing ions to migrate to either solution to ensure the system’s electrical neutrality. A voltmeter is a device that measures the flow of electric current between two half-reactions.
The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field.
Answer the following questions. [4]
(a) Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution :
Ag+(ag) + e– → Ag(s);E° = +0.80V
H+(aq) + e– → \(\frac{1}{2}\)H2(g); E° = 0.00V
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why?
(b) What does the positive value of standard electrode potential indicate?
(c) You have two half-cell reactions of an electrochemical cell are given below.
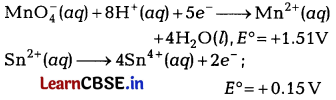
Imagine you are a member of scientific research working on electrochemistry project. Construct the redox equation from the two half-cell reactions and predict if this reaction favours formation of reactants or product shown in the equation.
Or
Give your comments on the following statement and justify your views.
(i) Single electrode potential cannot be determined.
(ii) An electrochemical cell act as electrolytic cell.
Answer:
(a) At the cathode, Ag+(ag) + e– → Ag(s)
reaction is feasible, because Ag+ ion has higher reduction potential, i.e. higher E° value.
(b) The positive value of standard electrode potential indicates that the element gets reduced more easily than H+ions and its reduced form is more stable than hydrogen gas.
(c) The reactions can be represented at anode and at cathode in the following ways.
At anode (oxidation):
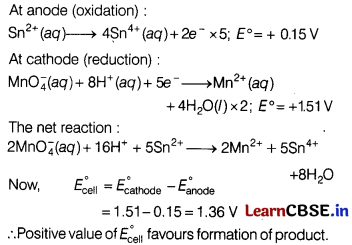
Or
(i) A single half-cell does not exist independently as reduction and oxidation occur simultaneously, therefore single electrode potential cannot be measured.
(ii) An electrochemical cell can be converted into electrolytic cell by applying an external opposite potential greater than its own electrical potential.[4]
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
Attempt any five of the following. [5]
(a) Why is the highest oxidation state is exhibited in oxo-anions of transition metals?
(b) Why the transition elements show high melting points?
(c) Why first ionisation enthalpy of Cr is lower than that of Zn?
(d) Explain the observation, although Cr3+ and Co2+ ions have same number of unpaired electrons but the magnetic moment of Cr3+ is 3.87 BM and that of Co2+ is 4.87 BM.
(e) What are the misch metals?
(f) Actinoids show large number of oxidation states than lanthanoids. Explain.
(g) What happens when a lanthanoid reacts with water?
Answer:
(a) The ability of oxygen to form multiple bonds with metal responsible for the high oxidation state of metal in oxoanions.
(b) Transition elements show high melting point, due to involvement of greater number of electrons in the interatomic bonding from (n- 1)d-orbitals in addition to ns-electrons.
Thus, they have large number of unpaired electron which are responsible for high strength of metallic bond.
(c) Ionisation enthalpy of Cr is less than that of Zn because Cr+ has stabled5 configuration. In case of zinc, electron comes out from completely filled 4s-orbital.
So, removal of electron from zinc requires more energy as compared to the chromium. (1)
(d) Magnetic moment of any metal ion can be decided on the basis of spin as well as orbital contribution of electron.’ Due to symmetrical electronic configuration, there is no orbital contribution in ion. However, appreciable orbital contribution takes place in Co2+ion.
(e) Misch metal is an alloy which consists of a lanthanoid metal (~95%) and traces of S, C, Ca and Al. It is used in Mg based alloy to produce bullets, shell and lighter-flint.
(f) Because in actinoids, 5f-orbitals are filled which are not as buried as lanthanoids and also participate in bonding to a greater extent besides 6d and 7s electrons.
(g) When a lanthanoid reacts with water, it forms hydroxide.
Ln + H2O → Ln(OH)3 + H2
![]()
Question 32.
(a) Explain why people feel weakness and discomfort in breathing at high altitude.
(b) Gautam have different solutions in two containers, 1M NaOH solution in one container and 1M Na2SO4 solution in second container. Help him to identify which container’s solution will have high boiling point.
(c) At 27°C, 25 mg of K2SO4 was dissolved in 2 L solution. Find out its osmotic pressure. Taking into consideration that K2SO4 has dissociated completely.
(R = 0.082 L atm K-1 mol-1) [5]
Or
(a) What do you understand by elevation of boiling point? How it is related to molality?
(b) Heptane and octane form an ideal solution. At 373 K, the vapour pressure of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35 g of octane ? [5]
Answer:
(a) At high altitude atmospheric pressure is low as compared to surface which causes difficulty in breathing. In this condition, people feel weakness and discomfort.
(b) As we know greater the value of van’t Hoff factor, higher will be the elevation in boiling point and hence, higher will be the boiling point of solution.
(c) Given : Mass of solute (w2) = 25 mg = 25 × 10-3 g
Volume of solution = 2 L
Temperature (T) = 273 + 27 = 300 K
R = 0.082 L atm K-1mol-1
∴ π = \(\frac{i \times W_2 \times R T}{V \times M_2}\)
π = \(\frac{3 \times 25 \times 10^{-3} \times 0.082 \times 300}{2 \times 174}\) (∵ i = 3)
= 0.0053 atm
Or
(a) Elevation of boiling point (∆Tb) On mixing, any non-volatile solute in solvent, the vapour pressure of solution decreases. As a result, boiling point of solution increases. The increase in boiling point of a solvent is known as elevation in boiling point (∆Tb). In mathematical form, ∆Tb = Ts – T0
where, Ts and T0 is boiling point of solution and solvent.
Relationship between elevation in boiling point and molality
Elevation in boiling point = Kb × molality where, Kb = molal elevation constant.
(b) Number of moles of octane
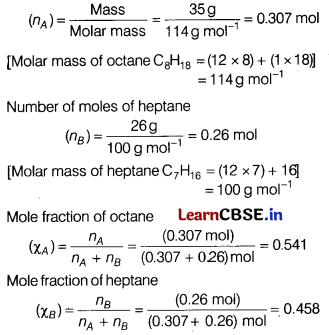
Vapour pressure of pure heptane (\(p_B^{\circ}\)) = 105.2 kPa
Vapour pressure of pure octane (\(p_A^{\circ}\)) = 46.8 kPa
In the mixture of 26.0 g heptane and 35.0 g octane
(i) Vapour pressure of heptane (pB) = p°B xB
= (105.2 kPa × 0.458)
= 48.18 kPa
(ii) Vapour pressure of octane
(pA) = P°A χA = (46.8 kPa × 0.541) = 25.32 kPa
(iii) Total vapour pressure of the mixture (p) = p°B + p°B
= 25.32+ 48.18= 735 kPa
Question 33.
Write structures of different isomers corresponding to the molecular formula, C3H9N. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid. [5]
Or
(a) Account for the following. [5]
(i) Methylamine is more basic than ammonia.
(ii) —NH2 group of aniline is acetylated before carrying out nitration.
(b) Arrange the following.
(i) In the decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH, C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2, C6H5N(CH3)2, (C2H5)2NH, CH3NH2
(iii) In increasing order of basic strength: Aniline, p-nitroaniline, p-toluidine.
Answer:
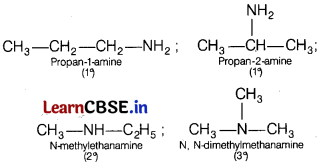
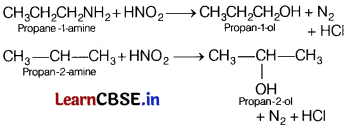
Only primary amines (1°) which are mentioned above will liberate nitrogen gas on treatment with nitrous acid.
Or
(i) Methylamine a stronger base than ammonia, since it has an electron donating group. Therefore, electron density on nitrogen atom increases. Hence, it is stronger base, whereas in ammonia electron donating group is absent.
(ii) In order to check the activation of benzene ring by amino group, first it is acetylated with acetic anhydride or acetyl chloride to form acetanilide which can be further nitrated easily by nitrating mixture.
(b) (i) C6H5NH2 > C6H5NHCH3 >C2H5NH2 > (C2H5)2NH
(The smaller pKb, the stronger is the base).
(ii) C6H5NH2 < C6H5N(CH3)2 < CH2NH2 < (C2H5)2NH
(On the basis of +R effect and accepting ability)
(iii) p-nitroaniline < aniline < p-toluidine
(On the basis of EWG or EDG attached to benzene ring.).