Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 3 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 3 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
Which one of the following pairs is the essential constituent of our food? [1]
(a) Nucleic acids and lipids
(b) Proteins and carbohydrates
(c) Proteins and nucleic acids
(d) Proteins and lipids
Answer:
(b) Proteins and carbohydrates
Among the given options, proteins and carbohydrates are the essential constituent of our food.
Question 2.
Consider the following reaction [1]
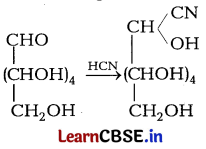
The given reaction confirms
(a) presence of primary alcoholic
(b) five — OH groups attached to
(c) different carbon atoms
(c) all six carbon atoms are linked in a straight chain
(d) the presence of a carbonyl group in
Answer:
(d) the presence of a carbonyl group in
In the given reaction, the molecule of hydrogen cyanide reacts with glucose to give cyanohydrin.
This reaction confirms the presence of a carbonyl group \(>C\) = O in glucose.
Question 3.
The compound which reacts fastest with Lucas reagent is (at room temperature) [1]
(a) butan-1-ol
(b) butan-2-ol
(c) 2-methyl propan-1-ol
(d) 2-methyl propan-2-ol
Answer:
(d) 2-methyl propan-2-ol
When Lucas reagent is treated with 1 2° and 3° alcohols, then turbidity appears. If turbidity is appeared immediately, then alcohol is tertiary. 2-methylpropan-2-ol is a tertiary alcohol. Hence, it reacts fastest with Lucas reagent.
![]()
Question 4.
Out of [TiF6]2-, [COF6]3-, Cu2Cl2 and [NiCl4]2- (At. no. of Ti = 22,Co = 27, Cu = 29 and Ni = 28 ), the colourless species are [1]
(a) [TiF6]2- and [CoF6]3-
(b) Cu2Cl2 and [NiCl4]2-
(c) [TiF6]2- and Cu2Cl2
(d) [CoF6]3- and [NiCl4]2-
Solution:
(c) [TiF6]2- and Cu2Cl2
Oxidation state of Ti in [TiF6]2- = Ti+4
= [Ne] 3s2 3p6 (no unpaired electrons)
Oxidation state of Cu in Cu2Cl2 = Cu+
= [Ar]3d10 (no unpaired electron)
∴ Both are colourless species as they do not have unpaired electrons.
Question 5.
Which of the following has the highest melting point but least solubility in a given solvent? [1]
(a) o-dichlorobenzene
(b) p-dichlorobenzene
(c) m-dichlprobenzene
(d) Chlorobenzene
Answer:
(b) p-dichlorobenzene
Due to symmetry, the molecule of p-dichlorobenzene fit closely in the lattice.
As a result, intermolecular forces are strongest in p-dichlorobenzene and, hence it has highest melting point and least solubility.
Question 6.
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code. [1]
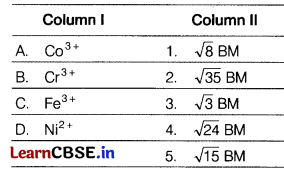
Codes

Solution:
![]()
The correct match is
A → 4, B → 5, C → 2, D → 1
Magnetic moment, µ = \(\sqrt{n(n+2)}\) BM
where, µ = magnetic moment
BM = Bohr Magneton (unit of µ)
n = number of unpaired electrons in d-orbital.
A. The electronic configuration of Co3+ is [Ar] 3d6.
Here, n = 4
µ = \(\sqrt{4(4+2)}\) = \(\sqrt{24}\)BM
B. The electronic configuration of Cr3+ is [Ar]3d3.
Here, n = 3
µ = \(\sqrt{3(3+2)}\) = \(\sqrt{15}\) BM
C. The electronic configuration of Fe3+ is [Ar]3d5.
Here, n = 5
µ = \(\sqrt{5(5+2)}\) = \(\sqrt{35}\) BM
D. The electronic configuration of Ni2+ is [Ar]3d8.
Here, n = 2
µ = \(\sqrt{2(2+2)}\) = \(\sqrt{8}\) BM
So, the correct option is (b).
![]()
Question 7.
A complex compound in which the oxidation number of a metal is zero, is [1]
(a) [Ni(CO)4]
(b) [Pt(NH3)4]Cl2
(c) K3[Fe(CN)6]
(d) K4[Fe(CN)6]
Answer:
(a) [Ni(CO)4]
CO is a neutral ligand, so the oxidation state of metal in metal carbonyl is always zero.
[Ni(CO)4]
x + (0 × 4) = 0
x = 0
Question 8.
Which of the following statements is not correct for amines? [1]
(a) All aliphatic amines are more basic than ammonia.
(b) Boiling point of 1° amine is lower than 2° and 3° amines.
(c) Aliphatic amines are soluble in water.
(d) Gabriel phthalimide synthesis cannot be used for the preparation of 2° and 3° amines.
Answer:
(b) Boiling point of 1° amine is lower than 2° and 3° amines.
Boiling point of 1° amine is higher than 2° and 3° amine due to the presence of two H-atoms attached directly with N, which results in greater extent of H-bonding in 1° amine.
Question 9.
Which of the following can be prepared using Gabriel phthalimide synthesis? [1]
(a) Primary aromatic amines
(b) Secondary amines
(c) Primary aliphatic amines
(d) Tertiary amines
Answer:
(c) Primary aliphatic amines
Primary aliphatic amines can be prepared by Gabriel phthalimide synthesis. Secondary and tertiary amines cannot be prepared by this method due to steric hindrance. Aromatic primary amines cannot be prepared because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Question 10.
Consider the following structures [1]
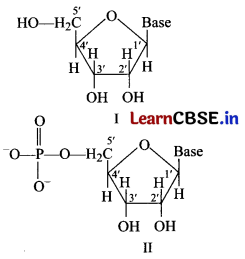
Identify I and II in the above structure and mark the correct option.

Answer:
(a) Structure I is a nucleoside and structure II is a nucleotide. A unit formed by the attachment of a base to 1′-position of sugar is known as nucleoside. In nucleosides, the sugar carbons are numbered as 1′, 2′, 3′ etc., in order to distinguish these from the bases. When nucleoside is linked to phosphoric acid at 5-position of sugar moiety, we get a nucleotide.
Question 11.
Consider the following graph, [1]
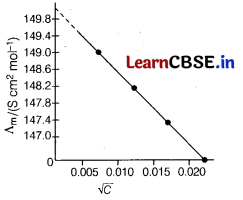
Here, the limiting molar conductivity is near about
(a) 149
(b) 150
(c) 149.4
(d) 147
Answer:
(b) 150
When concentration approaches zero, the molar conductivity is known as limiting molar conductivity. So, here limiting molar conductivity is near about 150.
Question 12.
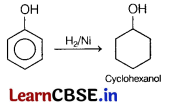
Phenol on reduction with H2 in the presence of Ni catalyst gives. [1]
(a) benzene
(b) toluene
(c) cyclohexane
(d) cyclohexanol
Answer:
(d) cyclohexanol
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is followed by a corresponding Reason (R). Use the following keys to chose the appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R) is true.
Question 13.
Assertion (A) In acylation reaction of amines, equilibrium shifts to the right hand side in the presence of pyridine.
Reason (R) In the presence of strong base, HCl is removed and reaction shifts toward the right hand side. [1]
Solution:
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
In acylation reaction of amines, equilibrium shifts to the right hand side in the presence of pyridine because it is a strong base which removes HCl, formed in the reaction.
Hence, both (A) and (R) are true and (R) is the correct explanation of (A).
Question 14.
Assertion (A) Cuprous ion (Cu+) has unpaired electrons, while cupric ion (Cu2+) does not.
Reason (R) Cuprous ion (Cu+) is colourless, whereas cupric ion (Cu2+) is blue in the aqueous solution. [1]
Solution:
(d) (A) is false, (R) is true.
Cu+ → [Ar]3d10 is diamagnetic because it has no unpaired electrons and is colourless.
Cu2+ → [Ar]3d9 is paramagnetic because it has one unpaired electron and is coloured.
![]()
Question 15.
Assertion (A) Separation of Zr and Hf is difficult.
Reason (R) Because Zr and Hf lie in the same group of the periodic table. [1]
Answer:
(b) Both (A) and (R) are true, (R) is not the correct explanation of (A).
Separation of Zr and Hf is difficult; it is not because they lie in the same group of periodic table. It is due to lanthanoid contraction which causes almost similar radii of both of them.
Question 16.
Assertion (A) Bromination of phenol takes place even in the absence of Lewis acid.
Reason (R) In phenol, OH-group attached to benzene ring has highly deactivating effect. [1]
Answer:
(c) (A) is true, (R) is false.
In case of phenol, the polarisation of bromine molecule takes place even in the absence of Lewis acid. It is due to the highly activating effect of OH-group attached to the benzene ring.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
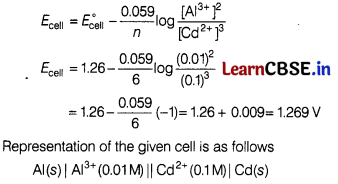
The value of E° for the cell is 1.260 V What is the value of Ecell ? [2]
2Al (s) + 3Cd2+ (0.1M) → 3 Cd(s) + 2Al3+(0.01M)
Represent the cell in which the above reaction takes place.
Solution:
Given, E°cell = 126 V
In this case, cell potential is given by the following equation.
Question 18.
Account for the following.
(a) Vitamin B and vitamin C are essential for us.
(b) Amino acids show amphoteric behaviour. [2]
Or
What happens when glucose is reacted with
(a) HI
(b) (CH3CO)2O [2]
Answer:
(a) Vitamins B and C are soluble in water which must be supplied regularly in diet because they are readily excreted in urine and cannot be stored (except vitamin B12) in our body.
(b) Amino acids contain both amino (—NH2) and carboxyl (—COOH) groups, thus they react with both acids and bases. Hence, amino acids are amphoteric in nature.
Or
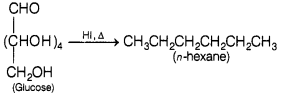
(a) When glucose is heated with HI for a long time, it forms n-hexane.
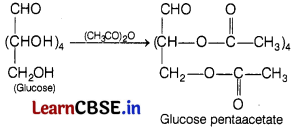
(b) When D-glucose is treated with (CH3CO)2CO, it gives pentaacetate.
Question 19.
Give reasons for the following. [2]
(a) The presence of —NO2 group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution reactions.
(b) p-dichlorobenzene has higher melting point than that of ortho or meta-isomer.
Answer:
(a) —NO2 shows -I and -M effect when present at ortho and para-position w.r.t. halogens. It has tendency to attract the electrons towards itself, thus they decrease the electron density between C — X (X = halogen) bond.
Therefore, —NO2 at ortho and para positions w.r.t. halogens in haloarenes increases the reactivity.
(b) p-dichlorbenzene has higher melting point than that of ortho or meta-isomers due to its symmetrical structure, which makes it a more compact compound and increases the melting point.
Question 20.
Explain how and why starch is different from
(a) glycogen
(b) cellulose [2]
Answer:
(a) Glycogen is a polysaccharide carbohydrate which is stored in animal body. It is also known as animal starch. Starch is a polymer of α-glucose and consists of two components amylose and amylopectin.
Amylose is a linear polymer of α-D-glucose. Both glycogen and amylopectin are branched polymers of α-D-glucose but glycogen is more highly branched than amylopectin. Amylopectin consists of 20-25 glucose units, while glycogen chain consists of 10-14 glucose units.
(b) Starch is a branched chain polymer of α-D-glucose, while cellulose is a linear polymer of β-D-glucose. Cellulose is present in the cell wall of plants, while starch is the storage material of plants.
Question 21.
For the complex [Cr(NH3)4Cl2]+, give
(a) structures of possible isomers.
(b) names of possible isomers. [2]
Answer:
(a) [Cr(NH3)4Cl2]+ shows geometrical isomerism and has two isomers cis and trans that can be represented as
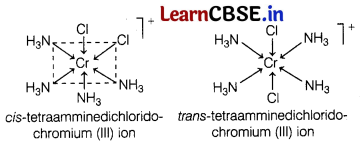
(b) Write the structures and names of the possible isomers of [Cr(NH3)4Cl2]+ complex.
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
Using valence bond theory, explain the following in relation to the paramagnetic complex [NiCl4]2-
(a) type of hybridisation.
(b) magnetic moment value.
(c) type of complex-inner or outer orbital complex. [3]
Answer:
(a) [Ni(Cl)4]2-
In case of [Ni(Cl)4]2-, Cl– ion is a weak field ligand. Therefore, it does not lead to the pairing of unpaired 3d-electrons. Therefore, it undergoes sp3-hybridisation. Since, there are 2 unpaired electrons in this case, it is paramagnetic in nature.
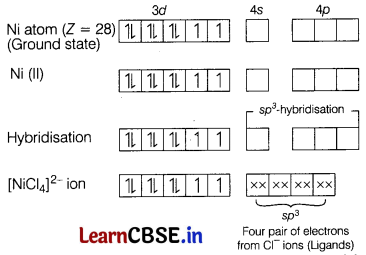
(b) Unpaired electrons = 2
Magnetic moment (µ) = \(\sqrt{n(n+2)}\)
= \(\sqrt{2(2+2)}\)
= 2.8 BM
(c) As Cl is weak field ligand, it prefers to form outer orbital complex.
![]()
Question 23.
Answer the following questions.
(a) State Henry’s law
(b) Mention two important applications of Henry’s law.
(c) Calculate the mass of compound (molar mass = 256 g mol-1) to be dissolved in 75 g of benzene to lower its freezing point by 0.48 K (Kf = 5.12 K kg mol-1). [3]
Answer:
(a) Henry’s law states that, the partial pressure of the gas in vapour phase (p) is directly proportional to the mole fraction of the gas (χ) in the solution.
P = KH ∙ χ
Here, KH = Henry’s constant.
Different gases have different KH values at the same temperature.
(b) Applications of Henry’s Law
- To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
- To minimise the painful effects of bends or decompression sickness in deep sea divers, oxygen diluted with less soluble helium gas is used as breathing gas.
(c) Given, that Kf = 5.12 K kg mol-1, W1 = 75g

Question 24.
An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid. Write the possible structure of the compound. [3]
Answer:
C = 69.77%, H = 11.63%, O = 100 – (69.77 + 11.63)
= 18.6%

Empirical formula of the given compound = C5H10O
Empirical formula mass = 5 × 12 + 10 × 1 + 1 × 16 = 86
n = \(\frac{86}{86}\) = 1
∴ Molecular formula = (C5H10O)1 = C5H10O
Since, it does not give Tollen’s test but gives positive iodoform test, hence it is a methyl ketone, i.e. have — COCH3 group. Since, on oxidation, it gives ethanoic acid and propanoic acid, it is pentan-2-one.

Question 25.
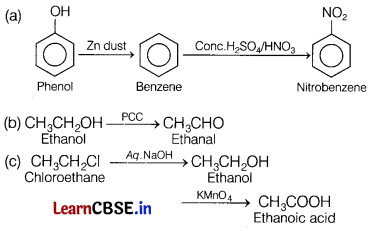
Write the equations for the following reaction.
(a) Phenol is treated with zinc dust and then with conc. HNO3 and H2SO4.
(b) Ethanol is treated with PCC.
(c) Chloroethane is treated with aq. NaOH and then followed by KMnO4. [3]
Answer:
Question 26.
(a) Identify the major product formed when hexanol is treated with SOCl2. Write the reaction involved.
(b) Why racemisation occurs in SN1 reactions? [3]
Or
(a) Name the possible product when chlorobenzene undergoes nitration reaction.
(b) Methyl chloride is hydrolysed more easily than chlorobenzene. Why? [3]
Answer:
(a) When alcohol reacts with thionyl chloride it produces good yield of alkyl chloride as by products formed are escapable gases.
C6H13OH + SOCl2 → C6H13Cl + SO2(g)↑+ HCl(g)↑
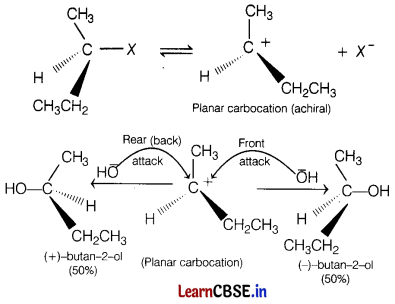
(b) In SN1 reactions, if the alkyl halide is optically active, the product obtained is a racemic mixture. The intermediate carbocation formed in slowest step being sp2-hybridised is planar (achiral) species.
Therefore, the attack of the nucleophile on it, can occur from both the faces (front and back) with equal ease, forming a 50:50 mixture of two enantiomers, i.e. the products with opposite configuration.
Or
(a)
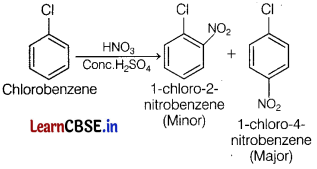
(b) Chlorobenzene is stabilised by resonance, so, it has a double bond character between C and Cl. Secondly, C is sp2-hybridised so more electronegative, thus Cl atom is not replaced easily.
While in methyl chloride, C— Cl has a single bond character which can be cleaved easily and hence, it is hydrolysed more easily than chlorobenzene.
Question 27.
Give reasons for the following observations.
(a) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
(b) NH2 group of aniline is acetylated before carrying out nitration.
(c) Higher aliphatic amines are not soluble in water. [3]
Answer:
(a) In aqueous solution, basic nature depends on + I-effect, H-bonding and steric effect.
The combined effect shows that (CH3)2 ∙ NH is more basic than (CH3)3 ∙ N as H-bonding is more in case of (CH3)2 ∙ NH than in (CH3)3N, which predominates over the stability due to +I- effect of three —CH3 groups.
(b) In order to check the activation of benzene ring by amino group, first it is acetylated with acetic anhydride or acetyl chloride in the presence of pyridine to form acetanilide which can be further nitrated easily by nitrating mixture.
(c) Higher aliphatic amines have larger hydrophobic chain due to which these amines are not able to show H-bonding. Hence, they are insoluble in water.
![]()
Question 28.
Data given below is for the reaction. [3]
2SO3(g) → 2SO2(g) + O2(g)
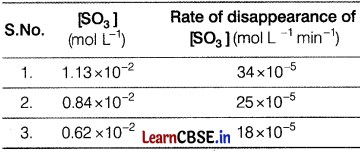
Determine for this reaction,
(a) order of reaction
(b) rate law of the reaction
(c) rate constant of the reaction.
Answer:
Let rate law of the reaction
r = k[SO3]α
from result (1) and (2) from the table.
34 × 10-5 = k(1.13 × 10-3)α
25 × 10-5 = k(0.84 × 10-2)α
Dividing both, \(\frac{34}{25}\) = \(\left(\frac{1.13}{0.84}\right)^\alpha\)
(1.36)1 = (1.35)α
α = 1.02
(a) Order of the reaction ≈ 1
(b) Rate law of the reaction, R = k [SO3].
(c) For k putting values in rate law from row 1 of table.
34 × 10-5 = k[1.13 × 10-2]1
k = 30.08 × 10-3
k = 3.0 × 10-2min-1
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
All of the physical processes that take place to keep a human body running are chemical processes. Nuclear reactions can lead to chemical damage, which the body may notice and try to fix. The nuclear reaction occurring in our bodies is radioactive decay. This is the change of a less stable nucleus to a more stable nucleus. Every atom has either a stable nucleus or an unstable nucleus, depending on how big it is and on the ratio of protons to neutrons. The ratio of neutrons to protons in a stable nucleus is thus around 1:1 for small nuclei (Z < 20). Nuclei with too many neutrons, too few neutrons, or that are simply too big are unstable. They eventually transform to a stable form through radioactive decay.
Wherever there are atoms with unstable nuclei (radioactive atoms), there are nuclear reactions occurring naturally. The interesting thing is that there are small amounts of radioactive atoms everywhere: in your chair, in the ground, in the food you eat, and yes, in your body. The most common natural radioactive isotopes in humans are carbon-14 and potassium-40. Chemically, these isotopes behave exactly like stable carbon and potassium. For this reason, the body uses carbon-14 and potassium-40 just like it does normal carbon and potassium; building them into the different parts of the cells, without knowing that they are radioactive. In time, carbon-14 atoms decay to stable nitrogen atoms and potassium-40 atoms decay to stable calcium atoms.
Chemicals in the body that relied on having a carbon-14 atom or potassium-40 atom in a certain spot will suddenly have a nitrogen or calcium atom. Such a change damages the chemical. Normally, such changes are so rare, that the body can repair the damage or filter away the damaged chemicals.
The natural occurrence of carbon-14 decay in the body is the core principle behind carbon dating. As long as a person is alive and still eating, every carbon-14 atom that decays into a nitrogen atom is replaced on average with a new carbon-14 atom. But once a person dies, he stops replacing the decaying carbon-14 atoms. Slowly the carbon-14 atoms decay to nitrogen without being replaced, so that there is less and less carbon-14 in a dead body.
The rate at which carbon-14 decays is constant and follows first order kinetics. It has a half – life of nearly 6000 years. So by measuring the relative amount of carbon-14 in a bone, archeologists can calculate when the person died. All living organisms consume carbon, so carbon dating can be used to date any living organism, and any object made from a living organism. Bones, wood, leather, and even paper can be accurately dated, as long as they first existed within the last 60,000 years. This is all because of the fact that nuclear reactions naturally occur in living organisms.
Answer the following questions.
(a) Why is carbon -14 radioactive, while carbon-12 is not? (Atomic number of carbon = 6)
(b) Researchers have uncovered the youngest known dinosaur bone, dating around 65 million years ago. How was the age of this fossil estimated?
(c) Which is the most common radioactive decays happening in human body?
(i) Carbon-14
(ii) Potassium-40
Suppose an organism has 20 g of carbon-14 at its time of death. Approximately how much carbon -14 remains after 10,320 years? (Given antilog 0.517 = 3.289) [4]
Or
Approximately how old is a fossil with 12 g of carbon -14, if it initially possessed 32 g of carbon -14? (Given log 2.667 = 0.4260) [4]
Answer:
(a) C-14 and C-12 have same number of protons (i.e. 6) but different number of neutrons (i.e. 8 and 6 respectively).
As C-14 atom has more neutrons than protons in its nucleus, therefore, its nucleus becomes unstable and shows radioactivity.
(b) Radioactive or carbon dating method is used to find out the age of fossils.
(c) The most common radioactive decays happening in human body is carbon-14.
(i) carbon-14 atoms decay to stable nitrogen atom and
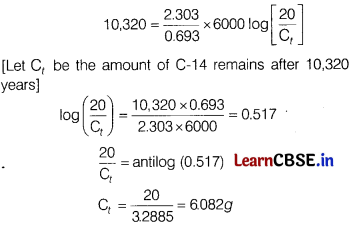
(ii) potassium-40 atoms decay to stable calcium atoms. Given, amount of C-14(C0) = 20g
Time = 10,320 years
According to first order, t1/2 = \(\frac{0.693}{k}\)
k = \(\frac{0.693}{6000}\)
[t1/2 = 6000 given in the passage]
For 1st order reactions t = \(\frac{2.303}{k}\)log\(\left[\frac{\mathrm{C}_0}{\mathrm{C}_t}\right]\)
On putting the value in first order equation, we get
Or
Given, initial amount of C-14 present in fossil C0 = 32g
Final amount of C-14, i.e. Ct = 12g.
Time (t) = ?
For 1st order reaction, k = \(\frac{0.693}{6000}\)
[half-life, i.e. 6000, is given in passage]
For 1st order reaction, t = \(\frac{2.303}{k}\)log\(\left(\frac{\mathrm{C}_0}{\mathrm{C}_t}\right)\) ….. (i)
Substituting the given value in Eqn. (i), we get
t = \(\frac{2.303}{0.693}\) × 6000 × log\(\frac{32}{12}\)
= 19939.4 log 2.667
= 19939.4 × 0.426
= 8494 years
![]()
Question 30.
Sourav was investigating the positive and negative deviation of solution from ideal solution.
He has taken pure acetone and chloroform whose vapour pressure at 328 K are 741.8 mm of Hg and 632.8 mm of Hg respectively.
Assuming that they form ideal solution over the entire range of composition, he obtained the experimental data observed for different compositions of mixture which is as follows

Answer the following question on the basis of above data. [4]
(a) First reading have zero values. What does it indicates?
Or
Why did Sourav collect different sets of readings?
(b) What will be the ptotal of first and last reading?
(c) What is the predicted behaviour of solution? Justify your answer.
Answer:
(a) Zero value indicates the pure component of the solution.
Or
To avoid error and maintain accuracy. In data collection, different sets of readings is gathered. [4]
(b) Ptotal = Pacetone + Pchloroform
In case of 1st reading,
Ptotal = 0 + 632.8 = 632.8
In case of last reading,
Ptotal = 322.7 + 257.7 = 580.9
The value of Ptotal decreases from 1st to last reading.
(c)
As the total calculated pressure ptota| is greater than the ptotal experimental or there is a dip in total pressure, then it indicates negative deviation from Raoult’s law.
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
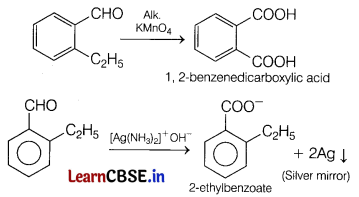
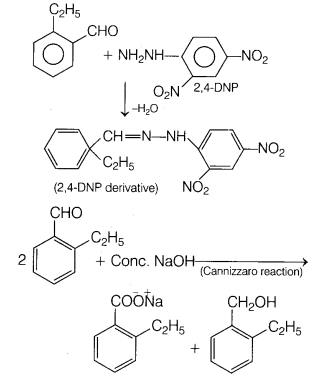
An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1, 2-benzene dicarboxylic acid.
Identify the compound and write all the reactions involved along with the name of the compounds involved in the reaction. [5]
Or
(a) An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C of molecular formula C6H7N. [5]
(i) Write the structures and IUPAC names of compound ‘A’, ‘B ‘ and ‘C ‘.
(ii) Give one method by which organic compound C can be prepared from B.
(iii) Write down the reaction in which A becomes its corresponding acid chloride.
(b) Complete the following reactions.
Answer:
Since, the compound gives 2,4-DNP derivative, it contains C = O group.
It reduces Tollen’s reagent, that means, the carbonyl compound is an aldehyde (—CHO group is present).
It gives Cannizzaro reaction, so it does not contain any α-hydrogen atom.
Thus, its possible structures are
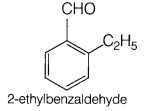
Since, the compound on vigorous oxidation gives 1,2-benzene dicarboxylic acid that means the two groups must be present at successive position.
The compound is
The reaction are as follows
Or
(a)
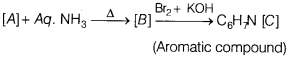
(i) Amine with molecular formula C6H7 is aniline. Hence, compound [C] is
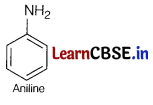
Therefore compound [B] must be benzamide, i.e
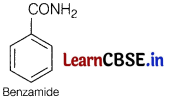
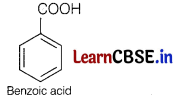
As compound [A] on heating with aqueous ammonia gives compound [B], so [A] must be benzoic acid,
The reactions involved are as follows
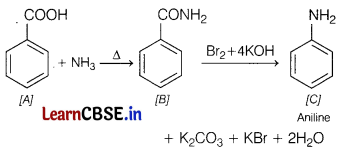
(ii) Compound ‘C’ of molecular formula C6H7N is result of Hofmann-bromamide degradation, hence [C] must be an amine and compound [B] must be an amide.
(iii)
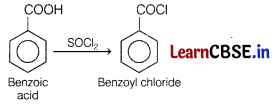
(b)
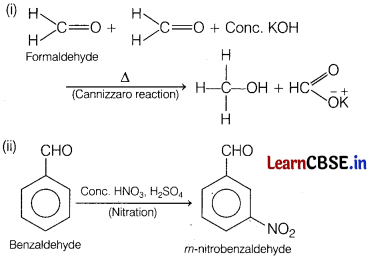
Question 32.
Attempt any five of the following. [5]
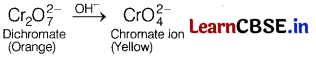
(a) Why orange colour of Cr2\(\mathrm{O}_7^{2-}\) ion changes to yellow when treated with an alkali?
(b) Why is the E° value for the Mn3+/Mn2+ couple is highly positive (+157 V) as compared to Cr3+ /Cr2+?
(c) Why actinoids exhibit a greater range of oxidation states than lanthanoids?
(d) Explain the observation, \(E_{M^{2+} / M}^0\) values are not regular for first row transition metals of 3d-series.
(e) What are the similarities between the chemistry of lanthanoids and that of actinoids?
(f) The transition metals (with the exception of Zn, Cd and Hg) are hard and have high melting and boiling point. Give reason.
(g) The ionisation enthalpies (first and second) in the first series of the transition elements are found to vary irregularly.
Answer:
(a) When orange solution containing Cr2\(\mathrm{O}_7^{2-}\) (dichromate ion) is treated with an alkali, a yellow solution of Cr\(\mathrm{O}_4^{2-}\) (chromate ion) is obtained.
(b) The comparatively high E° value for Mn3+ / Mn2+ is due to the fact that Mn2+(d5) is quite stable, whereas comparatively low value for Cr3+ / Cr2+ is because of the extra stability of Cr3+, compared to Cr+2, due to half-filled t2g orbitals. Therefore, Cr3+ cannot be reduced to Cr2+.
(c) Actinoids have lower ionisation energy and less effective nuclear charge, hence more number of valence electrons can take part in bond formation. It is due to the fact that 5f, 6d and 7s levels are of comparable energies. Therefore, actinoids exhibit +3,+ 4,+ 5,+ 6 and +7 oxidation states due to the participation of 5f, 6d and 7s-electrons in bond formation. Hence, actinoids exhibit greater range of oxidation states than lanthanoids.
(d) There is decreasing negative electrode potentials of M2+ /M in the first transition series due to increase in the sum of IE1 and IE2. It shows that in general, the stability of +2 oxidation state decreases from left to right. Exceptions are Mn and Zn in which the greater stability of +2 state for Mn is due to half-filled d-subshell (d5) and that of Zn is due to completely filled d-subshell (d10).
(e) Comparison of lanthanoids and actinoids. Similarities
- Both have mainly an oxidation state of +3.
- Both show magnetic and spectral properties.
(f) The transition metals (except Zn, Cd and Hg) are hard and have high melting and boiling points because transition elements display metallic properties. They show strong metallic bonding. Greater the number of unpaired electrons, stronger is the resultant metallic bonding.
(g) Ionisation enthalpy increases with increase in nuclear charge along each series. However, Cr has low first IE because loss of one electron gives stable electronic configuration (3d5). Zn has very high IE because electron has to be removed from 4s-orbital of the stable configuration (3d104s2).
Similarly, Cr and Cu show much higher values for second IE because the second electron has to be removed from the stable configuration of Cr+(3d5) and Cu+(3d10).
![]()
Question 33.
(a) E°cell for the given redox reaction is 2.7 IV
Mg(s) + Cu2+ (0.01 M) → Mg2+ (0.001 M) + Cu(s)
Calculate Ecell for the reaction.
(b) The resistance of 0.01 M NaCl solution at 25°C is 200Ω. The cell constant of the conductivity cell used is unity. What will the molar conductivity of the solution? [5]
Or
(a) A copper-silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not known. The cell potential when measured was 0.422 V
Determine the concentration of silver ions in the cell.
[Given, \(E_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{\circ}\) = +0.80 V,
\(E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\circ}\) = +0.34 V]
(b) How much electricity in terms of Faradays is required to produce 20 g of calcium from molten CaCl2 ?
(c) In the electrolysis of aqueous sodium bromide, there are two possible anodic reactions.
2H2O(l) → O2(g) + 4H+(ag) + 4e–, E° = – 1.23 V
2Br–(aq) → Br2(g) + 2e–, E° = – 1.08 V
Which reaction occurs at anode and why? [5]
Answer:
(a) For the reaction
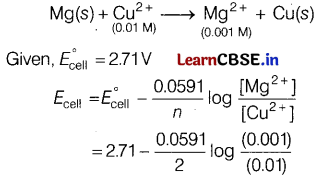
= 2.71 + \(\frac{0.0591}{2}\)log 10 = 2.74V
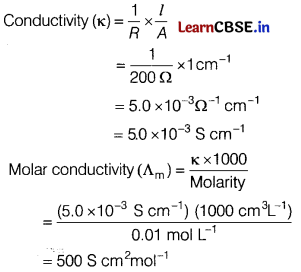
(b) Given : Resistance (R) = 200Ω
Molarity of NaCl solution = 0.01 M
Cell constant = \(\frac{l}{A}\) = 1 cm-1
Or
(a) Cell reaction is
Cu(s) + 2Ag+(ag) → Cu2+(ag) + 2Ag(s)
E°cell = ER – EL = + 0.80 – (+ 0.34) = 0.46 V
Number of electrons taking part, n = 2
By using Nernst equation,
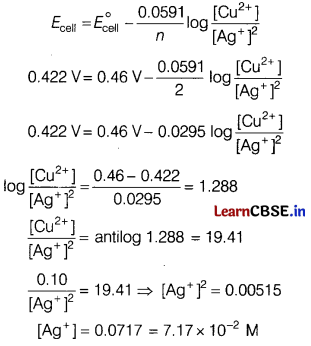
(b)
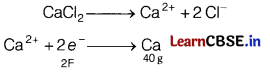
The electricity required to produce 40 g = 2 F
Therefore, electricity required to produce 20g
= (2 × 20)/ 40 = 1F
(c) In the electrolysis of NaBr,
2Br– (aq) → Br2 + 2e–
occurs at anode because it has lower reduction potential, and liberation of O2 requires overvoltage.