Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 11 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 11 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
Standard electrode potential for Sn4+/ Sn2+ couple +0.15 V and that for the Cr3+/ Cr couple is -0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be [1]
(a) 0.89
(b) 0.59
(c) -0.89
(d) -059
Answer:
(a) 0.89
The cell potential is given as,
E°cell = E°cathode – E°anode
= 0.15 – (-0.74)
= + 0.89 V
Question 2.
Which of the following does not give Fehling solution test? [1]
(a) PhCHO
(b) CH3CH2CH(Br)CH2CHO
(c) CH3CH2CHO
(d) CH3CH2CH2CHO
Answer:
(a) PhCHO
PhCHO do not give a positive test with Fehling’s solution because it lacks α-hydrogen and cannot form an enolate.
![]()
Question 3.
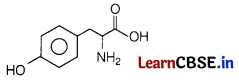
Which amino acid has phenolic —OH group as its backbone? [1]
(a) Glycine
(b) Leucine
(c) Serine
(d) Tyrosine
Answer:
(d) Tyrosine
Tyrosine is the amino acid which has phenolic —OH group as its backbone.
Question 4.
Consider the following reaction. [1]
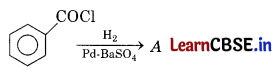
The product A is
(a) C6H5CHO
(b) C6H5OH
(c) C6H5COCH3
(d) C6H5Cl
Answer:
(a) C6H5CHO
Acid chloride reacts with H2 in the presence of Pd / BaSO4 to yield aldehyde. This reaction is called Rosenmund reduction.
Question 5.
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to [1]
(a) increase in number of ions
(b) increase in ionic mobility of ions
(c) 100% ionisation of electrolyte at normal dilution
(d) increase in both, i.e. number of ions and ionic mobility of ions [1]
Answer:
(b) increase in ionic mobility of ions
In strong electrolyte, number of ions remains constant so, equivalent conductance increases due to increase in ionic mobility.
Question 6.
The trend of which property is represented by the following graph? [1]
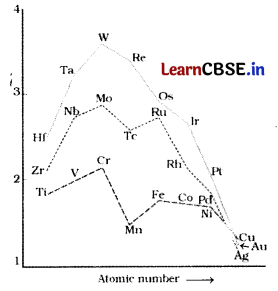
(a) ionisation enthalpy
(b) atomic radii
(c) enthalpy of atomisation
(d) melting point
Answer:
(d) melting point
The given figure depicts the melting points of transition metals belonging to 3d, 4d and 5d-series. The high melting points of these metals are attributed to the involvement of greater number of electrons from (n-1)d in addition to the ns-electrons in the interatomic metallic bonding.
Question 7.
In [Fe(CN)6]4- and [Fe(CN)6]3-, number of unpaired electrons respectively is [1]
(a) 4, 5
(b) 0, 1
(c) 5, 4
(d) 1, 2
Answer:
(b) 0, 1
Electronic configuration of
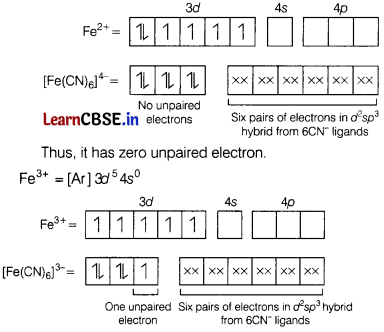
Thus, there is only 1 unpaired electron present in this complex.
Question 8.
Which of the following statements is not correct for amines? [1]
(a) Amines can be prepared by reduction of amides.
(b) Aromatic primary amines can be prepared from Gabriel phthalimide synthesis.
(c) Aryl nitro compound cannot be converted into amine using LiAlH4 in ether.
(d) In the Hofmann bromamide degradation, amine is formed with side products.
Answer:
(b) Aromatic primary amines can be prepared from Gabriel phthalimide synthesis.
Gabriel phthalimide synthesis is not useful for the preparation of aromatic primary amines because arylhalides do not undergo nucleophilic substitution with the anion formed by phthalimide.
![]()
Question 9.
The major product formed when phenol is heated with conc. nitric acid is [1]
(a) chlorotone
(b) picric acid
(c) methoxy benzene
(d) benzene
Answer:
(b) picric acid
When phenol is treated with conc. nitric acid, it gives picric acid.
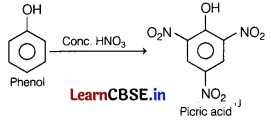
Question 10.
The complex ion which is diamagnetic in nature is [1]
(a) [CoF6]3-
(b) [NiCl4]2-
(c) [Ni(CN)4]2-
(d) [CuCl2]2-
Answer:
(c) [Ni(CN)4]2-
[Ni(CN)4]2- has dsp2-hybridisation ihere CN– is a strong field ligand.
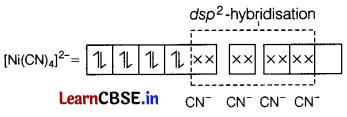
Since, all the electrons are paired, thus it is diamagnetic.
Question 11.
Major products formed by heating (CH3)3C—O —CH2— CH3 with HI are [1]
(a) (CH3 )3 C—I and CH3CH2OH
(b) (CH3)3C—OH and CH3CH2I
(c) (CH3)3 C—I and CH3CH2I
(d) (CH3)3C—OH and CH3CH2OH
Answer:
(b) (CH3)3C—OH and CH3CH2I
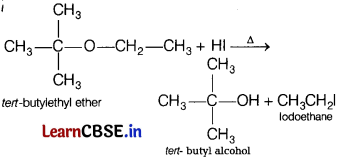
Question 12.
In Clemmensen reduction, carbonyl compound is treated with [1]
(a) zinc amalgam + HCl
(b) sodium amalgam + HCl
(c) zinc amalgam + nitric acid
(d) sodium amalgam + HNO3
Answer:
(a) zinc amalgam + HCl
Clemmensen reduction is used to convert carbonyl group to
![]()
group.
The general reaction is as follows
![]()
Zinc amalgam and HCl act as reagent in this reaction.
Direction (Q. Nos. 13-16) In the following questions an Assertion (A) is followed by a corresponding Reason (R). Use the following keys to choose the appropriate answer.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and ;R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 13.
Assertion (A) In alcohols, the boiling point decreases with decrease in the branching of the carbon chain.
Reason (R) There is the decrease in van der Waals’ forces between the number of carbon atoms with decrease in the surface area. [1]
Answer:
(d) (A) is false, but (R) is true.
The boiling point of alcohols and phenols increases with increase in the number of carbon atoms (increase in van der Waals’ forces). In alcohols, the boiling point decreases with increase of branching in carbon chain (because of decrease in van der Waal’s forces with decrease in surface area).
Question 14.
Assertion (A) tert-butyl bromide undergoes Wurtz reaction to give 2,2,3,3 -tetramethylbutane.
Reason (R) In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide. [1]
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
Question 15.
Assertion (A)Iron has higher enthalpy of atomisation than that of copper.
Reason (R) Lower the number of unpaired electron, higher will be the enthalpy of atomisation. [1]
Answer:
(c) (A) is true, but (R) is false.
Greater the number of unpaired electron, stronger will be bonding and thus enthalpy of atomisation will also
be more. Since, iron has more unpaired electron than copper therefore its enthalpy of atomisation is more.
Question 16.
Assertion (A) DNA undergoes replication.
Reason (R) DNA contains cytosine and thymine as pyrimidine base. [1]
Answer:
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
The genetic information of the cell is contained in the sequence of bases A, T, G and C in DNA molecule.
When a cell divides, DNA molecules replicate and make exact copies of themselves so that each daughter cell will have DNA identical to that of the parent cell.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
A coordination compound CoCl3 ∙ 4H2O precipitates silver chloride when treated with silver nitrate. This compound dissociates into two ions in solution. Write the structural formula of the compound and name it. [2]
Answer:
Formation of white precipitate with AgNO3 shows that atleast one Cl– ion is present outside the coordination sphere.
Moreover, only two ions are obtained in solution, so only Cl– is present outside the sphere. Thus, the formula of the complex is [CO(H2O)4 Cl2]Cl.
Its IUPAC name is tetraaquadichloridocobalt (III) chloride.
Question 18.
A 1.00 molal aqueous solution of trichloroacetic acid (CCl3COOH) is heated to its boiling point.
The solution has the boiling point 100.18° C. Determine the van’t Hoff factor for trichloroacetic acid.
(Kb for water = 0.512 K kg mol-1). [2]
Answer:
Consider the relation, ∆Tb = iKbm
Given, Molality of solution, m = 1.00 m
Boiling point of solution, Tb = 100.18° C = 37818 K
Boiling point of water (solvent)
T°b = 100.00° C = 373 K
∆Tb = Tb – T°b = 37318 K – 373 K = 0.18 K
∆Tb = i Kbm
0.18K = i × 0.512 K kg mol-1 × 1 mol kg-1
i = \(\frac{0.18 \mathrm{~K}}{0.512 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1} \times 1 \mathrm{~mol} \mathrm{~kg}^{-1}}\) = 0.35
![]()
Question 19.
Give reason for the following.
(a) Thionyl chloride method is preferred for preparing alkyl chloride from alcohols.
(b) Out of 1-bromopentane or 2-bromopentane, which will react faster towards SN2 reaction? [2]
Answer:
(a) Thionyl chloride (SOCl2) is preferred for preparation of alkyl chloride from alcohol, because the by-products we get in this reaction are escapable gases, thus we get good yield of alkyl-chloride, i.e.

(b) 1-bromopentane is a primary alkyl halide while 2-bromopentane is a secondary alkyl halide.
Since, primary alkyl halides are sterically less hindered therefore, 1 -bromopentane reacts faster towards SN2 reaction.
Question 20.
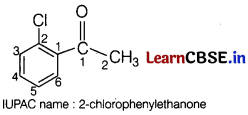
(a) Write the IUPAC name of the following : [2]
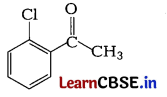
Answer:
(b) Which acid of given pair would you expect to be stronger acid?
F—CH2—COOH or Cl—CH2—COOH
Answer:
F— CH2 — COOH is a stronger acid than
Cl— CH2 — COOH because, higher the — I effect, stronger is the acid. The order of — I effect is
I < Br < CI < F.
Or
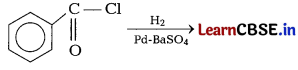
Predict the products of the following reactions. [2]
(a)
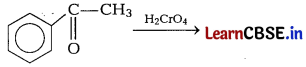
(b)
Answer:
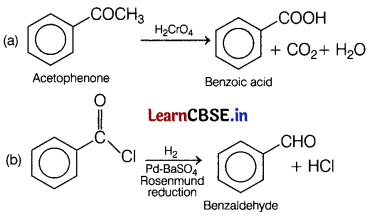
Question 21.
(a) Account for the following.
All the carbon-atoms in glucose are linked in a straight chain. [2]
Answer:
On prolonged heating with HI, glucose gives n-hexane which suggest that all the six carbon atoms in glucose are linked linearly.

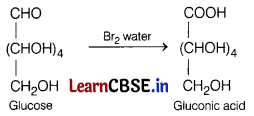
(b) What happens when D-glucose is treated with Br2 water?
Answer:
When D-glucose is treated with Br2 water, then gluconic acid is formed. The reaction involve is as follows
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
(a) State rate law and explain the difference between the average rate and instantaneous rate of a chemical reaction. [3]
Answer:
Rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may be or may not be same as the stoichiometric coefficient of the reacting species in a balanced chemical equation, e.g. For a general reaction,
aA + bB → cC + dD
Rate = k[A]a[B]b
Average rate of reaction It is defined as the change in the concentration of any one of the reactants or products over a long time interval.
Average rate of reaction \(=\frac{\text { Change in concentration }}{\text { Time interval }}\)
For a reaction, R → P
rav = –\(\frac{\Delta[R]}{\Delta t}\) = \(\frac{+\Delta[P]}{\Delta t}\)
Instantaneous rate of reaction It is defined as the rate of change in concentration of any one of the reactants or products at that particular instant of time.
For a reaction, R → P
rinst = –\(\frac{d[R]}{d t}\) = +\(\frac{d[P]}{d t}\)
(dt = very small interval of time)
(b) The rate constant for a zero order reaction in A is 0.0030 mol L-1s-1. How long will it take for the initial concentration of A to fall from 0.10 M to 0.075 M?
Answer:
For zero order reaction,
Rate constant, k = \(\frac{[R]_0-[R]}{t}\)
Given, [R]0 = 0.10 M, [R] = 0.075 M and k = 0.0030 mol L-1 s-1
∴ t = \(\frac{[R]_0-[R]}{k}\) = \(\frac{0.10-0.075}{0.0030}\) = 8.33 s
![]()
Question 23.
(a) Why does the voltage of a mercury cell remain constant during its operation? [3]
Answer:
The voltage of a mercury cell remains constant during its life as the overall reaction does not involve any ion in solution whose concentration can change during its life-time.
(b) The molar conductivity of substance ‘A’ is 6.3 × 103 S/m and ‘B’ is 1.8 × 10-16 S/m. Which of the two is most likely to be good conductor of electricity and why?
Answer:
‘A’ is the good conductor of electricity because it have high value of molar conductivity.
(c) The standard electrode potential for daniel cell is 1.1 V Calculate the standard Gibbs energy for the cell reaction. (F = 96500 C mol-1)
Zn(s) + Cu2+(ag) → Zn2+(ag) + Cu(s)
Answer:
Zn(s) + Cu2+ (aq) → Zn (aq) + Cu(s)
E° = +1.1V, ∆G°= ?, 1F = 96500 C mol-1, n = 2
∆G° = -nFE° = -2 × 96500 × 1.1
= -212300 J mol-1
= -212.3kJ mol-1
Question 24.
Draw the structure and name the product formed if the following alcohols are oxidised. (Assume that an excess of oxidising agent is used.) [3]
(a) CH3CH2CH2CH2OH
Answer:
![]()
(b) 2-butanol
Answer:
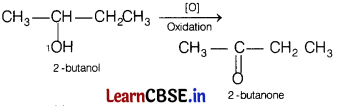
(c) 2-methyl-1-propanol
Answer:
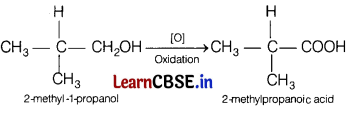
Question 25.
A ketone A (C4H8O) which undergoes a haloform reaction gives compound B on reduction. B on heating with sulphuric acid gives a compound C which forms mono-ozonide D. D on hydrolysis with zinc dust gives only acetaldehyde E.
Identify A, B, C, and D. Write an explanation for your answer. [3]
Answer:
(a)
Since, A gives haloform test, it must contain —COCH3 group.
Thus, its possible fomula is C2H5COCH3. On reduction, it gives 2-butanol, i.e. compound B which on dehydration with sulphuric acid gives 2-butene (C). Ozonolysis of 2-butene gives only acetaldehyde (E).
The equations involved are
A gives iodoform reaction as
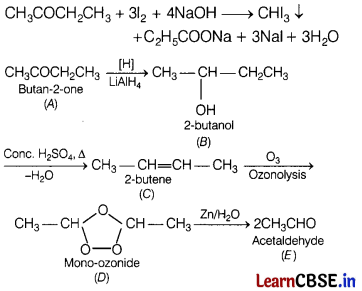
Or
An organic compound ‘A ‘ (C7H6O2) was prepared by the oxidation of compound B with alkaline KMnO4. Compound A on reduction with lithium aluminium hydride gets converted back to compound B. When the compound A is heated with compound B in the presence of H2SO4, it produces fruity smell of compound C.
Identify A, B and C and write the reaction of A with B to form C. [3]
Answer:
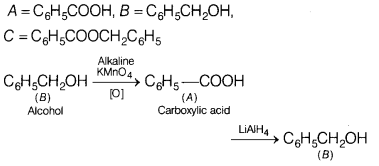
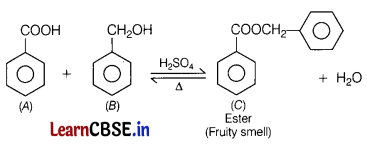
Question 26.
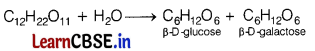
(a) Identify the products formed when lactose undergoes hydrolysis. [3]
Answer:
Lactose on hydrolysis gives β-D-galactose and β-D-glucose.
(b) Why the two strands of DNA are not identical but are complementary?
Answer:
Adenine is bound to thymine whereas guanine to cytosine. Due to this base pairing principle, the séquence of bases in one strand automatically fixed the sequence of bases in the other strand.
Thus, the two strands are not identical but are complimentary.
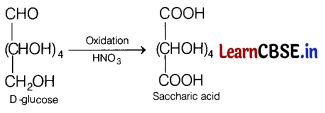
(c) Name the product which will be obtained when D-glucose reacts with cone. HNO3. Write the reactions involved.
Answer:
It is saccharic acid which is formed as per the reaction.
Question 27.
How would you differentiate between SN1 aid SN2 mechanism of substitution reactions? Give one example of each. [3]
Answer:
In SN1 mechanism of substitution reaction, the rate of reaction depends upon the concentration of only one reactant.
e.g. In the reaction between tert-butyl bromide and hydroxide ion to form tert-butyl alcohol, rate of reaction depends only on the concentration of tert-butyl bromide.
(CH3)3C—Br + OH– → (CH3)3C— OH + Br–
It involves the formation of carbocation intermediate and racemisation takes place.
In SN2 mechanism of substitution reaction , the rate of reaction depends upon the concentration of both the reactants, i.e. on both haloalkane and nucleophile, e.g. in the reaction between methyl chloride and
hydroxide ion to form methanol, rate of reaction depends on the concentration of both the reactants.
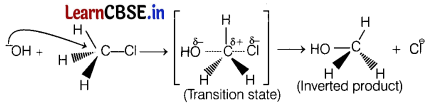
It involves the formation of transition state and inversion (Walden inversion) of configuration takes place.
Question 28.
Using valence bond theory, explain the following in relation to the complex [Cr(H2O)6]3+
(a) type of hybridisation.
(b) magnetic behaviour
(c) type of complex-inner or outer orbital complex. [3]
Answer:
[Cr(H2O)6]3+
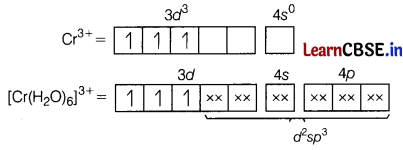
(a) d2sp3-hybridisation
(b) Paramagnetic (as three unpaired electrons are present.)
(c) μ = \(\sqrt{n(n+2)}\)
= \(\sqrt{3(3+2)}\) = \(\sqrt{15}\) = 3.87 BM
Inner orbital complex [as (n -1)d-orbital take part.]
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
Jorani performed an experiment to check the chemical kinetics of bacteria. Approximately 100 bacteria were placed in a flask containing nutrients, so that they can multiply. This experiment at 35°C gave the following results.
| Time (in min) | 0 | 15 | 30 | 45 | 60 |
| Number of bacteria | 100 | 200 | 400 | 800 | 1600 |
Answer the following questions on the basis of above data. [4]
(a) Which order of the reaction was followed in the multiplication of bacteria? Justify your answer.
Answer:
As the rate of the reaction increases with increase in concentration, the order of reaction is the first order.
Rate ∝ [No. of bacteria]
(b) Why did Jorani collect the reading at various time intervals?
Answer:
To maintain the accuracy and to avoid error in results, different sets of readings were taken.
(c) What would have been the value of rate constant at 30 minutes?
Answer:
For first order reaction,
Rate constant, k = \(\frac{-2.303}{t}\) log \(\frac{[A]_0}{[A]}\)
k = \(\frac{-2.303}{30}\)log\(\frac{100}{400}\)
k = – 0.076[log 1 – log 4]
k = – 0.076[-0.602] = 0.046 min-1
Or
What is the predicted half-life period (t1/2) of the reaction? [4]
Answer:
Half-life period (t1/2) for first order reaction is
t1/2 = \(\frac{0.693}{k}\)
For k = 0.304, t1/2 = \(\frac{0.693}{0.304}\) = 228 min.
Question 30.
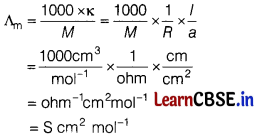
Molar conductivity can be expressed by an equation of type:
\(\Lambda_{\mathrm{m}}\) (Greek lambda) = \(\frac{\kappa}{C}\)
In the above equation, if \(\kappa\) is expressed in Sm-1 and the concentration C in mol m-3, then the units of \(\Lambda_{\mathrm{m}}\) are in S m2 mol-1.
Conductivity changes can frequently be useful for studying the reactions of metal complexes in solution. Molar conductivities (\(\Lambda_{\mathrm{m}}\)) are normally determined using 1 × 10-3 M solutions of the complexes.
The study of the effect of concentration and ionic size on the molar conductivities of electrolytes has been a concern to many researchers. Measurement of conductivity is an important electro-analytical method used to access the performance of battery. This is because it reveals the extent of the ion-solvent interactions in the solution.
It’s application in the high energy density batteries, photochemical cells, electrode deposition, wet electrolytic capacitor and electro-organic synthesis are among the most important application of electrolytic conduction. Agricultural and environmental soil assessments are renowned applications of salinity measurements. Molar conductance, \(\Lambda_{\mathrm{m}}\), is Known to be the conducting power of all the ions produced by one mole of electrolyte in a given solution. It is well known that the flow of electricity through a solution of electrolytes is due to the migration of ions when potential difference is applied between the two electrodes.
Molar conductance of solutions are affected by ionic mobility, concentration, temperature and inter-ionic interactions. It is important to note that molar conductivity of both strong and weak electrolytes increases with the depletion in dilution.
Answer the following questions. [3]
(a) Why does the conductivity of a solution decreases with dilution ?
Answer:
Conductivity of an electrolyte solution decreases with dilution because the number of ions per unit volume furnished by an electrolyte decreases with dilution.
Or
How is the unit at malar conductivity arrived ?
Answer:
(b) The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2mol-1. Calculate the conductivity of this solution. [3]
Answer:
Molar conductivity, \(\Lambda_m\) = 138.9 S cm2 mol-1
Molarity = 1.5 M; conductivity \(\kappa\) =?
Molar conductivity, \(\Lambda_m\) = \(\frac{\kappa \times 1000}{\text { Molarity }}\)
or \(\kappa\) = \(\frac{\Lambda_m \times \text { Molarity }}{1000}\)
= \(\frac{138.9 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1} \times 1.5 \mathrm{~mol} \mathrm{~L}^{-1}}{1000 \mathrm{~cm}^3 \mathrm{~L}^{-1}}\)
= 0.208 S cm-1
![]()
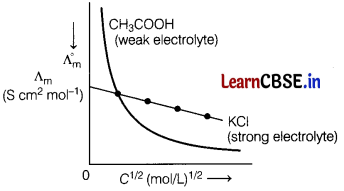
(c) X and Y are two electrolytes. On dilution molar conductivity of ‘X’ increases 2.5 times, while that of Y increases 25 times. Which of the two is a weak electrolyte and why? [3]
Answer:
Y is a weak electrolyte. On dilution, complete dissociation of weak electrolyte occurs and thus there is a steep increase in molar conductivity.
However, in case of strong electrolyte, it already dissociated completely therefore on dilution, the rise in conductivity is not very much, e.g.
Section E
(The following questions are long answer type and carry 5 internal choice.)
Question 31.
Attempt any five of the following.
(a) Why Cr2+ is a stronger reducing agent than Fe2+ in aqueous solution? [5]
Answer:
Cr2+([Ar] 3d4) changes to Cr3+ ([Ar]3d3), while Fe2+ ([Ar]3d6) changes to Fe3+ ([Ar] 3d5).
In aqueous medium, the configuration [Ar] 3d3 (or\(t_{2 g}^3\)) is more stable than the configuration [Ar] 3d5. Hence, Cr2+ is a stronger reducing agent.
(b) Why copper atom is considered as a transition element although it has completely filled d-orbitals (3d10)?
Answer:
Because it has incompletely filled d-orbitals in one of its common oxidation states. i.e. Cu2+ ([An]3d9).
(c) Why iron has higher enthalpy of atomisation than that of copper?
Answer:
Greater the number of unpaired electron, stronger will be bonding and thus, enthalpy of atomisation will also be more.
Since iron has more unpaired electron than copper therefore, its enthalpy of atomisation is more.
(d) Explain the observation, Zn2+ salts are white while Ni2+ salts are blue.
Answer:
Electronic configuration of Zn2+ = 3d10 4s0
![]()
Electronic configuration of Ni2+ = 3d84s0
![]()
Compounds that contains unpaired electrons are coloured, That’s why Zn2+ salts are white/colourless while Ni2+ salts are blue.
(e) What are the inner-transition elements?
Answer:
Inner-transition elements (or f-block elements) include lanthanoids (Z = 58 to 71) and actinoids (Z = 90 to 103). These elements have incomplete f-orbital in penultimate shell of their atoms.
(f) Write any one difference between the chemistry of lanthanoid and actinoid elements.
Answer:
Lanthanoids do not form oxocations while actinoids form oxocations such as \(\mathrm{UO}_2^{2+}\), \(\mathrm{PuO}_2^{2+}\), \(\mathrm{UO}_2^{+}\).
(g) How do you prepare K2MnO4 from MnO2?
Answer:
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
Question 32.
(a) Calculate the freezing point of solution when 1.9 g of MgCl2 (M = 95 g mol-1)was dissolved in 50 g of water, assuming MgCl2 undergoes complete ionisation.
(Kf for water = 1.86 K kg mol-1) [5]
Answer:
MgCl2 → Mg2+ + 2Cl–
1 mole of MgCl2 gives 3 moles of particles.
∴ i = 3
∆Tf = iKfm
Given, W1 = Weight of H2O (solvent) = 50 g
w2 = Weight of MgCl2 (solute) = 1.9 g
T°f = 273.15 K
Kf =1.86 K kg mol-1
M2 = Molar mass of solute = 95 g mol-1
∆Tf = \(\frac{i K_f \times 1000 \times W_2}{M_2 \times W_1}\)
= \(\frac{3 \times 1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1} \times 1000 \times 1.9 \mathrm{~g}}{95 \mathrm{~g} \mathrm{~mol}^{-1} \times 50 \mathrm{~g}}\)
∆Tf = 2.232K
Also, ∆Tf = T°f – Tf;
Tf = T°f – ∆Tf;
= 273.15 – 2.232 = 270.918 K
(b) (i) Out of 1 M glucose and 2 M glucose, which one has a higher boiling point and why? [5]
(ii) What happens when the external pressure applied becomes more than the osmotic pressure of the solution?
Answer:
(i) 2M glucose has higher boiling point because more the concentration, more is the elevation in boiling point.
(ii) When the external pressure applied becomes more than the osmotic pressure of solution, reverse osmosis takes place.
Or
(a) When 2.56 g of sulphur was dissolved in 100 g of CS2, the freezing point gets lowered by 0.383 K. Calculate the formula of sulphur (Sx). [5]
(Kf for CS2 = 3.83 K kg mol-1, Atomic mass of sulphur = 32 g mol-1)
Answer:
Given, Weight of solvent (w1) = 100g
Weight of solute (w2) = 2.56 g
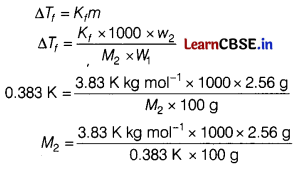
M2 = 256g/mol
Molecular mass = n × Atomic mass
n \(=\frac{\text { Molecular mass }}{\text { Atomic mass }}\) = \(\frac{256}{32}\) = 8
∴ Formula of sulphur is S8.
(b) Blood cells are isotonic with 0.9% sodium chloride solution. What happens if we place blood cells in a solution containing
(i) 1.2% sodium chloride solution?
(ii) 0.4% sodium chloride solution?
Answer:
(i) If we place the blood cells in a solution containing more than 0.9% (mass/volume) sodium chloride solution, water will flow out of the cells and they would shrink. This process is called plasmolysis.
(ii) If the salt concentration is less than 0.9% (mass/volume), then the water will flow into the cells and they would swell.
Question 33.
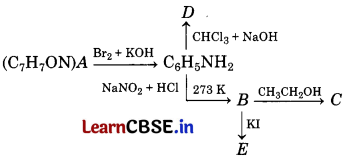
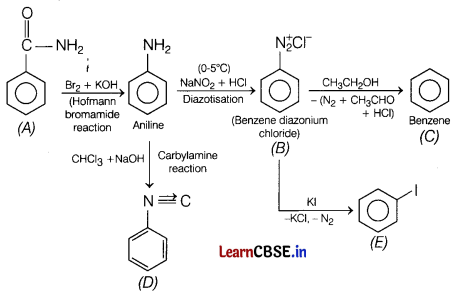
An aromatic compound ‘A’ of molecular formula C7H6ON undergoes a series of reactions as shown below.
Write the structures of A, B, C, D and E in the following reactions. [5]
Answer:
Or
(a) Account for the following.
(i) CH3NH2 is more basic than C6H5NH2.
(ii) Cyclohexanone forms cyanohydrin and gives good yield but 2,2,6-trimethyl- cyclohexanone does not.
(iii) Aromatic diazonium salts are more stable than aliphatic diazonium salt.
Answer:
(i) In C6H5NH2, the lone pair of electron on N is delocalised over the benzene ring and becomes less available for protonation. The lone pair on CH3NH2 is localised and much more susceptible to protonation. Thus, CH3NH2 is more basic.
(ii)
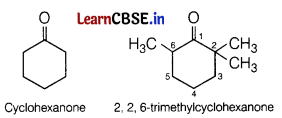
In 2, 2, 6-trimethylcyclohexanone, total three methyl groups are present at a-position with respect to the ketonic ![]()
group.
Therefore, these groups cause steric hindrance during the nucleophilic attack of CN– ion. So, cyanohydrin is not formed.
Due to the absence of methyl group in cyclohexanone, there is no steric hindrance and cyanohydrin is formed.
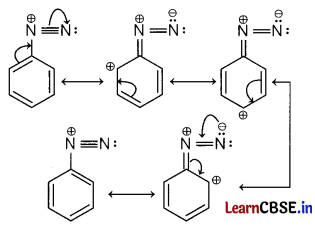
(iii) Diazonium salts of aromatic amines are more stable than those of aliphatic amines due to the delocalisation of the positive charge on the benzene ring as shown by resonating structure.
(b) Arrange the following in the
(i) increasing order of their pKb values.
C6H5NH2, C2H5NH2, C6H5NHCH3
(ii) increasing order of their boiling point.
C2H5NH2, C2H5OH, (CH3)3N
Answer:
(i) C2H5NH2 < C6H5NHCH3 < C6H5NH2
(ii) (CH3)3N < C2H5NH2 < C2H5OH