Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 10 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 10 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
The electrolytic purification of copper can be carried out in an apparatus similar to the one shown below. [1]
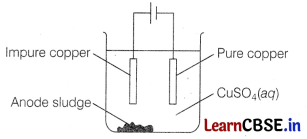
The half equation for the reaction at the anode is
(a) Cu(s) + 2e– → Cu2+
(b) Cu(s) → Cu2+ + 2e–
(c) Cu2+ + 2e– → Cu
(d) Cu2+ → Cu + 2e–
Answer:
(b) Cu(s) → Cu2+ + 2e–
Impure copper acts as anode. So, the reaction involved at anode is Cu(s) → Cu2+ + 2e–.
Question 2.
The major product formed when ethanol is heated with HI and red P is [1]
(a) ethyl iodide
(b) ethane
(c) ethylene
(d) ether
Answer:
(b) ethane
When C2H5OH is heated with HI and red R it forms ethane.
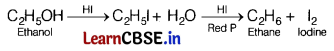
Question 3.
A magnetic moment of 1.73 BM will be shown by which among the following, [1]
(a) [CU(NH3)4]2+
(b) [Ni(CN)4]2-
(c) TiCl4
(d) [CoC]6]4-
Answer:
(a) [CU(NH3)4]2+
Magnetic moment, µ is related to number of unpaired electrons as
µ = \(\sqrt{n(n+2)}\) BM ⇒ (1.73)2 = n(n + 2)
On solving, we get n = 1
Thus, the complex/compound having one unpaired electron exhibit a magnetic moment of 1.73 BM.
(a) In [CU(NH3)4]2+, Cu2+ = [Ar] 3d9
![]()
(Although in the presence of strong field ligand NH3, the unpaired electrons gets excited to higher energy level but it still remains unpaired.)
(b) In [Ni(CN)4]2-, Ni2+ = [Ar] 3d8
![]()
But CN– being strong field ligand pair up the unpaired electrons and hence, in this complex, number of unpaired electrons = 0
(c) In [TiCl4], Ti2+ = [Ar] no unpaired electron.
(d) In [CoCl6]4-, Co2+ = [Ar] 3d7
![]()
It contains three unpaired electrons.
Thus, [Cu(NH3)4]2+ is the complex that exhibits a magnetic moment of 1.73 BM.
![]()
Question 4.
Which one of the following alkyl halides will undergo SN1 reaction most readily? [1]
(a) (CH3)3C—F
(b) (CH3)3 C—Cl
(c) (CH3)3C—Br
(d) (CH3)3C—I
Answer:
(d) (CH3)3C—I
All the given compounds are tertiary alkyl halides but the bond formed between carbon and iodine (C—I) bond is the weakest bond due to large difference in the size of carbon and iodine. So, (CH3)3C— I gives SN1 reaction most readily. In other words, iodine is a better leaving group.
Question 5.
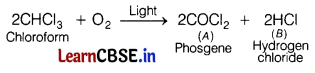
Consider the following reaction,
2CHCl3 + O2 → A + B
The products A and B of above reaction respectively are [1]
(a) CO2 and HCl
(b) COCl2 and HCl
(c) CO and HCl
(d) None of the above
Answer:
(b) COCl2 and HCl
The products A and B in the given reaction are COCl2 and HCl.
Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas, carbonyl chloride (phosgene).
Question 6.
The maximum oxidation state shown by Mn in its compound is [1]
(a) +4
(b) + 5
(c) +6
(d) + 7
Answer:
(d) + 7
In excited state,
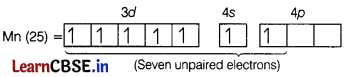
Hence, maximum oxidation state exhibited by Mn is+7 which is in KMnO4.
Question 7.
Which of the following statements is not correct for aldehydes?
(a) Compounds having —CHO group can reduce Fehling solution.
(b) Formaldehyde is the least polar among ethanal and propanal due to lowest electron density.
(c) Butanal is more polar than ethoxyethane.
(d) Aldehydes on oxidation gives 1° alcohols. [1]
Answer:
(b) Formaldehyde is the least polar among ethanal and propanal due to lowest electron density.
The incorrect statement is option (b). It is because formaldehyde is the most polar among ethanal and propanal due to lowest electron density.
Question 8.
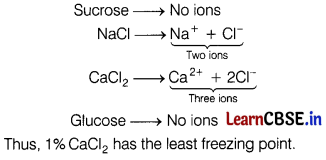
Which has the least freezing point?
(a) 1% Sucrose
(b) 1% NaCl
(c) 1% CaCl2
(d) 1% Glucose [1]
Answer:
(c) 1% CaCl2
Depression of freezing point is a colligative property (depends only upon the number of particles of solutes). Thus, the compound which produces maximum ions has the least freezing point.
∵ Concentration is same.
Question 9.
Match the laws with expressions:
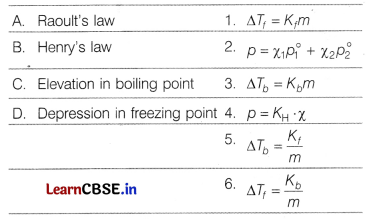
Codes
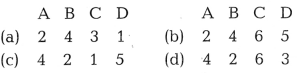 [1]
[1]
Answer:
(a)
![]()
The correct match is A – 2, B – 4, C – 3, D – 1.
Question 10.
The reaction by which benzaldehyde is converted in benzyl alcohol, is [1]
(a) Fittig reaction
(b) Cannizzaro reaction
(c) Wurtz reaction
(d) Aldol condensation
Answer:
(b) Cannizzaro reaction
The given reaction is called Cannizzaro reaction and involves oxidation and reduction of same compound.
Question 11.
Identify (i), (ii) and (iii) in the following diagram. [1]
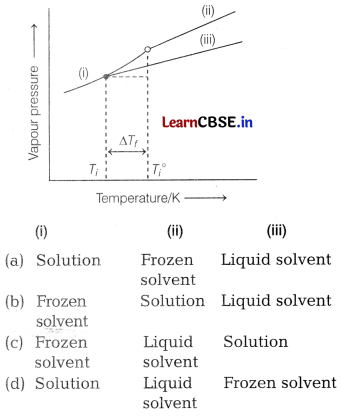
Answer:
(c) (i) Frozen solvent – (ii) Liquid solvent – (iii)Solution
In the given graph, the curve (ii) shows the variation of vapour pressure of pure liquid solvent with temperature. The addition of non-volatile , solute decreases the vapour pressure of solution 1 and therefore curve (iii) shows the variation of vapour pressure of solution with temperature. Curve (i) corresponds to the vapour pressure of frozen solvent at different temperature.
Thus, (c) is the correct option.
Question 12.
What is (are) the number(s) of unpaired electrons in the square planar [Pt(CN)4]2- ion? [1]
(a) Zero
(b) 1
(c) 4
(d) 6
Answer:
(a) Zero
Outer electronic configuration of Pt is 5d9, 6s1.
Outer electronic configuration of Pt2+ is 5d8.
As CN– is strong field ligand, so pairing will take place.
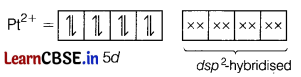
So, there is no unpaired electron in the square planar [Pt(CN)4]2- ion.
Hence, it contains zero unpaired electrons.
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is followed by a corresponding Reason (R). Use the following keys to choice the appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct explanation of (A).
(c) (A) is correct, (R) is false.
(d) (A) is false, (R) is true.
Question 13.
Assertion (A) MeNH2 is a weaker base than MeOH.
Reason (R) N is less electronegative than O, lone pair of electrons on N is more easily available for donation in MeNH2. [1]
Answer:
(d) (A) is false, (R) is true.
MeNH2 is a stronger base than MeOH because N is less electronegative than O and lone pair of electrons on N is more easily available for the donation in MeNH2.
Hence, (A) is false but (R) is true.
![]()
Question 14.
Assertion (A) Bond angle in ether is slightly less than the tetrahedral angle.
Reason (R) There is a repulsion between the two bulky (—R) groups. [1]
Answer:
(d) (A) is false, (R) is true.
(A) is false but (R) is true.
Correct Assertion Bond angle in ether is slightly more than the tetrahedral angle.

Question 15.
Assertion (A) Glucose does not form the hydrogen bisulphite addition product.
Reason (R) Glucose is not so reactive to form the product with NaHSO3. [1]
Answer:
(c) (A) is correct, (R) is false.
Glucose does not form the hydrogen bisulphite addition product because it has cyclic structure in which —CHO group is not free to react. Therefore,
(A) is true, but (R) is false.
Question 16.
Assertion (A) CO is stronger complexing reagent than NH3.
Reason (R) CO is a good σ-donor and a good π-acceptor while NH3 can form only σ-bonds and no π-bonds. [1]
Answer:
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
As CO is a good donor and a good π-acceptor ligand, there exists a back bonding in CO complexes in which CO accepts an appreciable amount of electron density from the filled d-orbitals of metal atom into their empty π or π* orbitals.
These π interactions increase the value of ∆0. Whereas NH3 can form only σ-bonds and no π-bonds with metals, therefore CO is a better complexing reagent than NH3.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
(a) Write the reaction when 2-propanone is reduced with zinc amalgam. [2]
Answer:
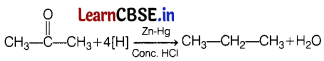
(b) Write the name of this reaction. The IUPAC name of the product formed in above reaction.
Answer:
The above reaction is called as Clemmensen reduction reaction. The IUPAC name of the product formed in above is propane.
Question 18.
(a) Name the suitable haloalkane and reagent from which methyl isocyanide can be prepared. [2]
Answer:
It can be done by treating the methyl bromide with alcoholic silver cyanide.
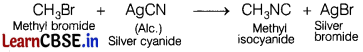
(b) Out of ethyl bromide and ethyl iodide, which one undergoes SN2 reaction faster?
Answer:
As I– ion is a better leaving group than Br– ion, therefore iodides are more reactive than bromides.
Therefore, CH3—CH2—I is more reactive than CH3—CH2 — Br towards SN2 reaction and hence, CH3—CH2—I would undergo SN2 reaction faster than CH3—CH2—Br.
Question 19.
Calculate ∆rG° and log KC for the following reaction at 298 K. [2]
2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd(s)
[Given: E°cell] = + 0.34 V, F = 96500 C mol-1]
Answer:
∆rG °= -nFE°cell, n = 6
= -6 × 96500 C / mol × 0.34 V
= -196860 J/mol or – 196.860 kJ/mol
∵ E°cell = 0.059 V/n × logKc
logKc = \(\frac{0.34 \mathrm{~V} \times 6}{0.059 \mathrm{~V}}\) = 34.5762
Question 20.
Explain how and why [2]
(a) elevation of boiling point of 1M KCl solution is nearly double than that of 1 M sugar solution?
Answer:
Elevation in boiling point is directly proportional to ‘i’. ∆Tb ∝ i. Now as given in the question, elevation of boiling point of 1 M KCl solution is nearly double than that of 1 M sugar solution. It is because KCI
being ionic, dissociates into K+ and Cl– and therefore, it’s van’t Hoff factor is 2 whereas for sugar van’t Hoff factor is 1 as it does not undergo such a dissociation.
(b) Al2(SO4)3 exhibits largest freezing point depression than KCl and C6H12O6?
Answer:
Al2(SO4)3(aq) \(\rightleftharpoons\) 2 Al3+ + 3\(\mathrm{SO}_4^{2-}\)
(Total ions = thus, i = 5)
Whereas for KCI the value of i is 2 and glucose do not ionise.
Hence, the value of i is largest for Al2(SO4)3. So, Al2(SO4)3 will exhibit largest freezing point depression.
Question 21.
Account for the following. [2]
(a) A solution containing non-volatile solute have higher boiling point than the pure solvent.
Answer:
Boiling point of a solution containing a non-volatile solute is higher than the pure solvent because the solute particles decreases the vapour pressure of the solution since the solute particles have no vapour pressure of their own. So, the solution is heated to a higher temperature to become equal to atmospheric pressure for the boiling process to take place.
(b) Elevation of boiling point is a colligative property.
Answer:
Elevation of boiling point is a colligative property because it depends upon the number of solute particles present in a solution.
Or
What happens when blood cells are placed in [2]
(a) water ?
(b) concentrated solution ?
Answer:
(a) When blood cells are placed in water, then water enters the cell by osmosis. Blood cells will swell.
(b) When blood cells are placed in concentrated solution, then water moves out of the cell faster than it comes in. This results in shrinking of blood cells.
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
(a) Identify the major product formed when chlorobenzene undergoes Friedel-Crafts acylation.
Name the reagent which is used to carry out the reaction. [3]
Answer:
To carry out Friedel-Crafts acylation of chlorobenzene, reagent anhyd. AlCl3 is used.
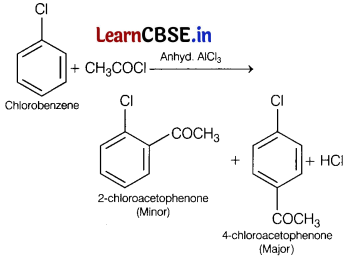
(b) Why does the presence of nitro group (—NO2) at o/p-positions increase the reactivity of haloarenes towards nucleophilic substitution reaction?
Answer:
The reactivity of haloarenes towards nucleophilic substitution reactions can be increased by the presence of an electron withdrawing group (—NO2)at ortho and pa/a-positions. This is because, —NO2 group at ortho and para-positions withdraw electron density from the benzene ring and thus, facilitates the nucleophilic attack on haloarenes.
The carbanion thus formed is stabilised through resonance. The negative charge appeared at ortho and para position with respect to the halogen substituent is stabilised by —NO2 group.
Or
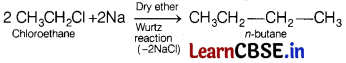
(a) Name the possible haloalkane which will yield n-butane on their reaction with sodium.
Write the reactions involved. [3]
Answer:
Chloroethane will yield n-butane on their reaction with sodium which is as follows
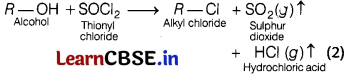
(b) Thionyl chloride method is preferred for preparing alkyl chloride from alcohols. Why?
Answer:
Thionyl chloride (SOCl2)is preferred for preparation of alkyl chloride from alcohol, because the by-products we get in this reaction are escapable gases, thus we get good yield of aikyl-chioride i.e.
Question 23.
Answer the following questions. [3]
(a) What are pseudo first order reaction? Explain the kinetics of acidic hydrolysis of ester.
Answer:
Pseudo first order reaction The reaction which is bimolecular but has order one, is called pseudo first order reaction, e.g. acidic hydrolysis of ester.
![]()
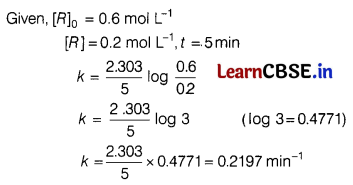
(b) In a first order reaction, the concentration of the reactant is reduced from 0.6 mol L-1 to 0.2 mol L-1 in 5 min. Calculate the rate constant of the reaction.
Answer:
Rate constant, k = \(\frac{2.303}{t}\) log \(\frac{[R]_0}{[R]}\).
(c) For a reaction R → P, half-life (t1/2) is observed to be independent of the concentration of reactants. What is the order of reaction ?
Answer:
As given reaction is independent of initial concentration of reactants. Thus, it follows first order reaction.
![]()
Question 24.
Write the equations for the following reaction. [3]
(a) Benzyl chloride is treated with aq. NaOH.
Answer:
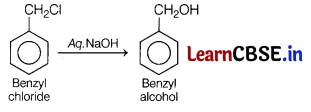
(b) Methyl magnesium bromide is reacted with propanone followed by hydrolysis.
Answer:
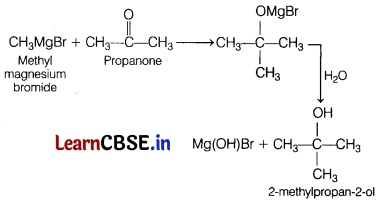
(c) Propene undergoes acid hydrolysis.
Answer:
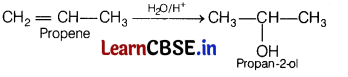
Question 25.
Using valence bond theory, explain the following in relation to the complex K4[Mn(CN)6]. [3]
(a) IUPAC name
(b) Type of hybridisation
(c) Magnetic behaviour
Answer:
(a) K4[Mn(CN)6]
IUPAC name Potassium i exacyanomanganate (II)
(b) Let the oxidation state of Mn be x.
1 × 4 + x + (-1)6 = 0 ⇒ x = +2
Thus, in this complex Mn is present a Mn2+.
Mn2+ = [Ar]3d5
[CN– being strong field ligand, causes pairing]
K4[Mn(CN)6]
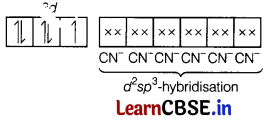
⇒ Low spin, inner orbital complex.
Thus, the structure of this complex is octahedral,
(c) Magnetic behaviour Paramagnetic
(as one unpaired electron is present in the above orbital diagram).
Question 26.
An organic compound A (C3H80) on treatment with copper at 573 K gives B.
B does not reduce Fehling solution but gives a yellow ppt. of compound C with I2 / NaOH. Deduce the structures of A, B, and C. [3]
Answer:
Compound B gives a yellow ppt. with I2/NaOH, i.e. positive iodoform test and not reduce Fehling solution, it means that it contains — COCH3 (methyl ketone) group, and is a ketone. Moreover, B is obtained by the oxidation of A, thus A must be a 2° alcohol.
(As only 2° alcohol give ketones on oxidation with Cu at 573 K). Hence, the structure of compound A is
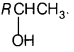
Comparing with the given molecular formula gives R = CH3. Thus, compound A is
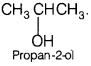
The reactions are as follows
Question 27.
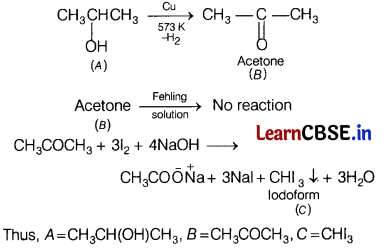
Compound A having molecular formula C4H10O, is found to be soluble in concentrated sulphuric acid. It does not react with sodium metal or potassium permanganate.
On heating with excess of HI, it gives a single alkyl halide. Deduce the structure of compound A and explain all the reactions. [3]
Answer:
- As compound A does not react with sodium metal or potassium permanganate, it cannot be an alcohol.
- As compound Adissolves in cone. H2SO4, it may be an ether.
- As compound A on heating with excess of HI, gives a single alkyl halide, therefore compound A must be a symmetrical ether.
- The only symmetrical ether having molecular formula C4H10O is diethyl ether. Thus, compound A is diethyl ether, CH3—CH2—O—CH2—CH3.
Question 28.
How can reducing and non-reducing sugars be distinguished? Mention the structural features characterising reducing sugars. [3]
Answer:
Reducing sugars The sugars which reduce Fehling solution and Tollen’s reagent are called reducing sugars, e.g. all monosaccharides containing free
![]()
groups are reducing sugars. Thus, the presence of free aldehydic or ketonic group is main feature of reducing sugars.
Non-reducing sugars The sugars which do not reduce Fehling solution or Tollen’s reagent are called non-reducing sugars, e.g. sucrose.
In non-reducing sugars, reducing groups of monosaccharides, i.e. aldehydic or ketonic groups are bonded.
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow)
Question 29.
Polysaccharides may be very large molecules. Starch, glycogen, cellulose, and chitin are examples of polysaccharides.
Starch is the stored form of sugars in plants and is made up of amylose and amylopectin (both polymers of glucose). Amylose is soluble in water and can be hydrolysed into glucose units breaking glycocidic bonds, by the enzymes a-amylase and β-amylase. It is straight chain polymer. Amylopectin is a branched chain polymer of several D-glucose molecules. 80% of amylopectin is present in starch.
Plants are able to synthesise glucose and the excess glucose is stored as starch in different plant parts, including roots and seeds. The starch that is consumed by animals is broken down into smaller molecules, such as glucose. The cells can then absorb the glucose.
Glycogen is the storage form of glucose in human and other vertebrates, and is made up of monomers of glucose. It is structural support to the cell. Wood and paper are mostly cellulosic in nature.
Like amylose, cellulose is a linear polymer of glucose. Cellulose is made up of glucose monomers that are linked by bonds between particular carbon atoms in the glucose molecule.
Every other glucose monomer in cellulose is flipped over and packed tightly as extended long chains.
Answers the following questions. [4]
(a) Glycogen is known as animal starch. In animals it is stored in which part?
Answer:
In animals, glycogen is stored in liver.
(b) What type of linkage is present between monosaccharides to form long chain polysaccharides?
Answer:
Glycosidic linkage joins monosaccharides and polysaccharides.
(c) What is glycogen and explain how glycogen is different from starch?
Answer:
Glycogen is a polysaccharide carbohydrate which is stored in animal’s body. It is also known as animal starch. Starch is a polymer of α-glucose and consists of two components amylose and amylopectin Amylose is a linear polymer of α-D-glucose. Both glycogen and amylopectin are branched polymers of α-D-glucose but glycogen is more highly branched than amylopectin. Amylopectin consists of 20-25 glucose units while glycogen chain consists of 10-14 glucose units.
Or
What is essentially the difference between the a -form of glucose and β-form of glucose? Explain. [4]
Answer:
The α-glucose and β-glucose form differ from each other in orientation of —OH group at C-1.
Moreover, the α-form is obtained by cystallisation from concentrated solution of glucose at 303 K while β-form is obtained by crystallisation from hot and saturated solution at 371 K.
Question 30.
Riya performed an experiment on chemical kinetic of a reaction. She took the A and B reactants and changed their concentrations. The initial rates for different initial concentrations of the reactants rate are given below. [4]
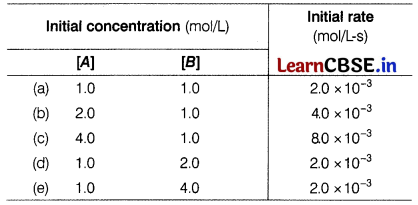
The reaction involved is A + B → C + D
Now, answer the following questions according to the above given reaction and its experimental data.
(a) What will be the rate constant?
Answer:
Since, r = k[A]
Then, rate constant,
k = \(\frac{r}{[A]}\) = \(\frac{2 \times 10^{-3}}{1.0}\) = 2 × 10-3s-1
Or
What is the predicted t1/2 of the reaction with respect to A? [4]
(b) Why did Riya collect different sets of readings?
(c) What is the order of reaction with respect to A and B ?
Answer:
t1/2 for first order reaction,
t1/2 = \(\frac{0.693}{k}\)
= \(\frac{0.693}{2 \times 10^{-3}}\) [from above answer]
= 3465 s
(b) To maintain the accuracy and to avoid the error in data collection, different sets of readings were taken.
(c) Rate of reaction α [concentration]”, where, n is the order of reaction.
Given reaction, A + B → C + D
Suppose the given reaction is of order p with respect to A and q with respect to B.
The rate of formation of C and D can also be written as
- 2.0 × 10-3 = k[A]p[B]q = k(1)p(1)q ……(i)
- 4.0 × 10-3 =k(2)p(1)q …….(ii)
- 2.0 × 10-3 = k(1)p(2)q ……..(iii)
From Eqs. (i) and (ii), we get
\(\frac{4.0 \times 10^{-3}}{2.0 \times 10^{-3}}\) = \(\frac{k(2)^p(1)^p}{k(1)^p(1)^q}\) ⇒ (2)1 = 2p
Hence, p = 1
From Eqs. (i) and (iii), we get
\(\frac{2.0 \times 10^{-3}}{2.0 \times 10^{-3}}\) = \(\frac{k(1)^p(2)^q}{k(1)^p(1)^q}\) ⇒ 1 = 2q ⇒ 20 = 2q
Hence, q = 0
∴ The order of reaction with respect to A is first order, i.e. r ∝ [A] and with respect to 6 is zero order, i.e. r ∝ [B]0.
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
(a) Chromium metal is electroplated using an acidic solution containing CrO3 according to the following equation.
CrO3 (aq) + 6H+ + 6e– → Cr(s) + 3H2O
Calculate how many grams of chromium will be electroplated by 24000 coulombs. How long will it take to electroplate 1.5 g chromium using 12.5 A current?
[Atomic mass of Cr = 52 g mol-1, 1 F = 96500 C mol-1] [5]
Answer:
Given : Charge (Q) = 24000 C and
current (i) = 12.5A.
Mass of chromium (Cr) deposited = 1.5 g
Molar mass of (Cr) = 52 g mol-1
and 1 F = 96500 C mol-1
∵ 6 × 96500 C charge deposit Cr = 52 g
∴ 24000 C charge deposit Cr = \(\frac{52 \times 24000}{6 \times 96500}\)
= \(\frac{1248000}{6 \times 96500}\)
Mass of chromium deposited = 2.16 g
Time taken to deposit 1.5 g Cu by 12.5 ampere current.
⇒ m = Zlt
m = \(\frac{E}{96500}\)l.t
1.5 = \(\frac{52}{6 \times 96500}\) × 12.5 × t(sec)
t = \(\frac{1.5 \times 6 \times 96500}{52 \times 12.5}\)
t = 1336.16 s
t =22.2 minutes
(b) Write applications of Kohlrausch’s law.
Answer:
Kohlrausch’s law of independent migration of ions states that the limiting molar conductivity of an electrolyte can be expressed as the sum of the individual contributions of the anion and the cation of the electrolyte, e.g.
![]()
Applications of Kohlrausch’s law: Some important applications of Kohlrausch’s law are as follows
- The molar conductivity of weak electrolytes at infinite dilution can be calculated by using Kohlrausch’s law.
- Molar conductivity of weak electrolyte (like acetic acid) can be calculated at a given concentration.
- Degree of dissociation of weak electrolyte can be calculated from dissociation constant.
- Solubility of sparingly soluble salts can be calculated as
S = \(\frac{\kappa \times 1000}{\Lambda_m^{\circ}}\)(mol/L)
Or
(a) The molar conductivity of A is 46.1 S cm/mol and that of B is 0.025 S cm/mol. at 0.02 M. Which of the two is most likely to be methanoic acid? [5]
Answer:
Molar conductivity of weak electrolyte is more than the strong electrolyte. Therefore, A would be methanoic acid.
(b) How many moles of mercury will be produced by electrolysing 10 M Hg(NO3)2 solution with a current of 2.00 A for three hours?
[Hg(NO3)2 = 200.6 g mol-1]
Answer:
Given, current = 2A,
Time = 3h = 3 × 60 × 60s
m = Zlt
= \(\frac{E}{96500}\)
= \(\frac{200.6 \times 2 \times 3 \times 60 \times 60}{2 \times 96500}\)lt
= 22.45g
Number of moles
= \(\frac{22.45}{200.6}\) = 0.112 mol
(c) Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution.
Ag+ (aq) + e– → Ag(s); E° = 0.80 V
H+(ag) + e– → \(\frac{1}{2}\)H2(g); E° = 0.00 V
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why?
Answer:
Electrolysis of an aqueous solution of AgCl.
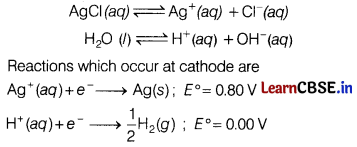
From ∆G°= -nFE°, more negative AG° will be more will be the feasibility of that reaction.
Since, E° is higher for Ag + (ag) → Ag, feasibility of occurrence of this reaction at cathode is higher.
![]()
Question 32.
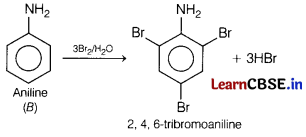
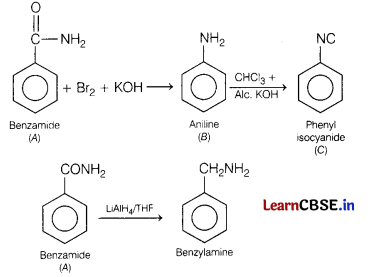
An aromatic compound ‘A’ on heating with Br2 and KOH forms a compound ‘B ‘ of molecular formula C6H7N, which on reacting with CHCl3 and alcoholic KOH produces a foul smelling compound ‘C’. [5]
Write the chemical equation involved in the formation of A, B and C. Explain the reaction of B with Br2/H2O. Also, write the equation for reduction of A with LiAlH4/THF.
Answer:
Since, compound ‘A’ on heating with Br2 and KOH forms a compound ’S’ which further reacts with CHCl3 and ale. KOH producing afoul smelling compound ‘O’.
Thus, compound ‘C ’ is an aromatic isocyanide and compound ‘S ’ is aniline with formula C6H5NH2 and compound ‘A ’ must be benzamide.
Or
(a) A compound (X) has molecular formula as C3H7NO. It gives the following reactions. Hydrolysis of (X) gives an amine (Y) and carboxylic acid (Z). Amine (Y) with Hinsberg reagent forms water insoluble product. [5]
(i) Identify X, Y and Z.
(ii) Write down the reaction for the formation Z and Y from X.
(iii) Give the test for compound Y which shows it is insoluble in water.
Answer:
(i) Compound [X] C3H7NO must be an amide because it gives carboxylic acid and an amine on hydrolysis.
![]()
Amine [Y] must be a secondary amine as it produces an insoluble compound on treatment with Hinsberg’s reagent, i.e. benzene sulphonyl chloride.
Also, as only formic acid gives a positive silver mirror test, hence acid [Z] must be HCOOH.
(ii)The reaction involved in the formation of Y and Z from X is as follows
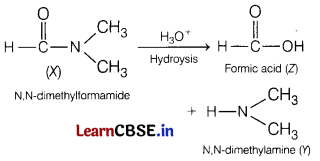
(iii) Reaction of compound Y, i.e.
N, N-dimethylamine reacts with benzene sulphonyl chloride to yield insoluble product as shown.
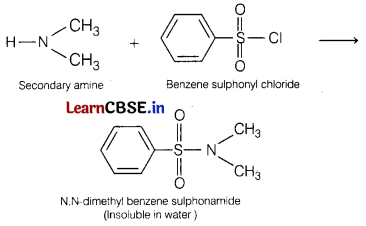
(b) Account for the following.
(i) Which compound is soluble in water, N, N-dimethylamine or formic acid? Why?
(ii) Write down the reaction for formic acid on treatment with Tollen’s reagent giving a positive silver mirror test.
Answer:
(i) Both the given compounds are soluble in water because both can form hydrogen bonds with water.
(ii)

Question 33.
Attempt any five of the following. [5]
(a) Which element of transition series has highest second ionisation enthalpy?
Answer:
Copper has highest second ionisation enthalpy because second electron is to be removed from fully-filled d-subshell.
Cu = 3d104s1
(b) Why does copper not replace hydrogen from acids?
Answer:
\(E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^0\) = + 0.34V
Positive standard reduction potential shows that copper will not displace hydrogen gas on treatment with mineral acid as Cu is less reactive than hydrogen.
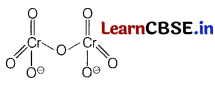
(c) Draw the structure of dichromate ion.
Answer:
Dichromate ion (Cr2\(\mathrm{O}_7^{2-}\))
(d) How will you obtain Na2Cr2O7 from Na2CrO4 ?
Answer:
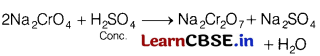
(e) Why scandium (21) is a transition element but not calcium (20)?
Answer:
Sc(21) with electronic configuration [Ar] 3d14s2 have incompletely filled d-orbitals, whereas Ca(20) with electronic configuration [Ar] 4s2 does not have electrons in d-orbitals. Thus, Sc(21) is a transition element.
(f) Explain the observation, the second and third rows of transition elements resemble each other much more than they resemble the first row.
Answer:
Due to lanthanoid contraction, the atomic radii of the second and third rows of transition elements is almost same. So, they resemble each other much more as compared to first row elements and show similar characteristics.
(g) What happens when KMn04 is treated with oxalic acid in acidic medium ?
Answer:
KMnO4 oxidises oxalic acid to CO2 and itself changes to Mn2+ ions which is colourless because KMnO4 acts as an oxidising agent.