CBSE Class 12 Chemistry Lab Manual
- Introduction to Basic Laboratory Equipment
- Surface Chemistry
- Exp-2.1 : To prepare colloidal solution (sol) of starch.
- Exp-2.2 : To prepare a colloidal solution of gum.
- Exp-2.3 : To prepare colloidal solution (or sol) of egg albumin.
- Exp-2.4 : To prepare ferric hydroxide, [Fe(OH)3] sol.
- Exp-2.5 : To prepare aluminium hydroxide, [Al(OH)3] sol.
- Exp-2.6 : To prepare colloidal solution of arsenious sulphide, [As2 S3].
- Exp-2.7 :To study the dialysis of starch sol containing sodium chloride through a cellophane or parchment paper.
- Exp-2.8 : Compare the precipitation values of sodium chloride, barium chloride and aluminium chloride for arsenious sulphide sol.
- Exp-2.9 : To study the effectiveness of different common oils (castor oil, cotton seed oil, coconut oil, kerosene oil, mustard oil) in forming emulsions.
- Exp-2.10 : To compare the effectiveness of a number of emulsifying agents in forming emulsions.
- Surface Chemistry Viva Questions with Answers.
- Chemical Kinetics
- Exp-3.1 : To study the effect of concentration on the rate of reaction between sodium thiosulphate and hydrochloric acid.
- Exp-3.2 : To study the effect of change in temperature on the rate of reaction between sodium thiosulphate and hydrochloric acid.
- Exp-3.3 : To study the reaction rate of reaction of iodide ions with hydrogen peroxide at different concentrations of iodide ions.
- Exp-3.4 : To study the reaction rate of the reaction between potassium iodate (KIO3) and sodium sulphite (Na2S03) using starch solution as indicator.
- Chemical Kinetics Viva Questions with Answers.
- Thermochemistry
- Exp-4.1 : Determine the calorimeter constant (W) of calorimeter (polythene bottle).
- Exp-4.2 : Determine the enthalpy of dissolution of given solid copper sulphate (CuS04.5H20) in water at room temperature.
- Exp-4.3 : Determine the enthalpy of neutralisation of hydrochloric acid with sodium hydroxide solution.
- Exp-4.4 : Determine the enthalpy change during the interaction (hydrogen bond formation) between acetone and chloroform.
- Thermochemistry Viva Questions with Answers.
- Electrochemistry
- Exp-5.1 : To set up simple Daniell cell and determine its emf .
- Exp-5.2 : To set up simple Daniell cell using salt bridge and determine its emf .
- Exp-5.3 : To study the variation of cell potential in Zn | Zn2+ || Cu2+ | Cu cell with change in concentration of electrolytes (CuS04 and ZnS04) at room temperature.
- Electrochemistry Viva Questions with Answers.
- Chromatography
- Exp-6.1 : Separate the coloured components present in the mixture of red and blue inks by ascending paper chromatography and find their Rf values .
- Exp-6.2 : Separate the coloured components present in the given grass/flower by ascending paper chromatography and determine their Rf values .
- Exp-6.3 : Separate Co2+ and Ni2+ ions present in the given mixture by using ascending paper chromatography and determine their Rf values .
- Chromatography Viva Questions with Answers.
- Preparation of Inorganic Compounds
- Exp-7.1 : To prepare a pure sample of ferrous ammonium sulphate (Mohr’s salt), [FeSO4 . (NH4)2 SO4.6HO20] .
- Exp-7.2 : To prepare a pure sample of potash alum (Fitkari), [K2SO4.Al2 (SO4)3. 24H20] .
- Exp-7.3 : To prepare a pure sample of the complex potassium trioxalatoferrate(III), Kg[Fe(C2O4)3l . 3H20 .
- Preparation of Inorganic Compounds Viva Questions with Answers.
- Preparation of Organic Compounds
- Exp-8.1 : To prepare a sample of acetanilide from aniline.
- Exp-8.2 : To prepare a sample of dibenzalacetone.
- Exp-8.3 : To prepare a sample of p-nitroacetanilide from acetanilide .
- Exp-8.4 : To prepare 2-naphthol aniline or phenyl-azo-β-naphtholdye .
- Preparation of Organic Compounds Viva Questions with Answers.
- Tests for the Functional Groups Present in Organic Compounds
- Tests of Carbohydrates, Fats and Proteins in Pure Samples and Detection of Their Presence in Given Food Stuffs
- Exp-10.1 : To study some simple tests of carbohydrates .
- Exp-10.2 : To study some simple tests of oils and fats .
- Exp-10.3 : To study some simple tests of proteins .
- Exp-10.4 : To detect the presence of carbohydrates, fats and proteins in the following food stuffs : Grapes, potatoes, rice, butter, biscuits, milk, groundnut, boiled egg .
- Tests of Carbohydrates, Fats and Proteins in Pure Samples and Detection of Their Presence in Given Food Stuffs Viva Questions with Answers.
- Volumetric Analysis
- Exp-11.1 : Prepare 250 ml of M/10 solution of oxalic acid from crystalline oxalic acid .
- Exp-11.2 : Prepare 250 ml of a N/10 solution of oxalic acid from crystalline oxalic acid .
- Exp-11.3 : Preparation of 250 ml of M/20 solution of Mohr’s salt .
- Exp-11.4 : Preparation of 250 ml of N/20 solution of Mohr’s salt .
- Exp-11.5 : Prepare M/20 solution of ferrous ammonium sulphate (Mohr’s salt). Using this solution find out the molarity and strength of the given KMn04 solution.
- Exp-11.6 : Prepare a solution of ferrous ammonium sulphate (Mohr’s salt) containing exactly 17.0 g of the salt in one litre. With the help of this solution, determine the molarity and the concentration of KMnO4 in the given solution.
- Exp-11.7 : Prepare M/20 ferrous ammonium sulphate (Mohr’s salt) solution. Find out the percentage purity of impure KMnO4 sample 2.0 g of which have been dissolved per litre .
- Exp-11.8 : Determine the equivalent mass and number of molecules of water of crystallisation in a sample of Mohr’s salt, FeSO4(NH4)2 SO4 . nH20. Provided KMnO4.
- Exp-11.9 : Prepare M/50 solution of oxalic acid. With its help, determine 50 the molarity and strength of the given solution of potassium permanganate (KMnO4).
- Exp-11.10 : Find out the percentage purity of impure sample of oxalic acid. You are supplied M/100 KMnO4 solution.
- Exp-11.11 : The given solution has been prepared by dissolving 1.6 g of an alkali metal permanganate per litre of solution. Determine volumetrically the atomic mass of the alkali metal. Prepare M/20 Mohr’s salt solution for titration.
- Exp-11.12 : Determine the percentage composition of a mixture of sodium oxalate
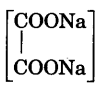 and oxalic acid
and oxalic acid 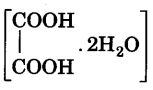 . Provided M/100 KMnO4 solution.
. Provided M/100 KMnO4 solution. - Exp-11.13 : You are provided with a partially oxidised sample of ferrous sulphate (FeSO4.7H20) crystals. Prepare a solution by dissolving 14.0 g of these crystals per litre and determine the percentage oxidation of the given sample. Given M/100 KMnO4 solution.
- Exp-11.14 : Calculate the percentage of Fe2+ ions in a sample of ferrous sulphate. Prepare a solution of the given sample having strength exactly equal to 14.0 g/litre. Provided M/100 KMnO4 .
- Exp-11.15 : Prepare N/20 Mohr’s salt solution. Using this solution, determine the normality and strength of the given potassium permanganate solution.
- Exp-11.16 : Prepare N/20 solution of oxalic acid. Using this solution, find out strength and normality of the given potassium permanganate solution .
- Exp-11.17 : Determine the percentage purity of the given sample of oxalic acid. Ask for your requirement .
- Exp-11.18 : Determine the percentage composition of a mixture of sodium oxalate
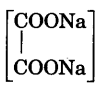 and oxalic acid
and oxalic acid 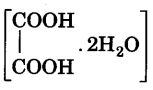 . Provided N/20 KMnO4 .
. Provided N/20 KMnO4 . - Exp-11.19 : Determine the equivalent mass and number of molecules of water of crystallisation in a sample of Mohr’s salt FeSO4 (NH4)2 SO4.nH20. Provided N/20 KMnO4.
- Volumetric Analysis Viva Questions with Answers.
- Chemistry Qualitative Analysis